Oligodendrocytes (ODs) are a type of glial cell that produce myelin sheath to allow for the insulation of segments of neuronal axons. This enables high velocity signal transduction, which is essential for the propagation of action potentials along the axon. ODs also contribute to neuroplasticity and provide trophic support to neurons. Each OD can extend its processes to multiple axons and has a great capacity to rapidly renew its myelin sheath. As a result, ODs have a high metabolic rate and are highly vulnerable to oxidative stress. The latter is also partly due to ODs having relatively low levels of anti-oxidative enzymes. As a consequence, ODs are highly susceptible to proinflammatory molecules produced during inflammation and respond by producing low quality myelin. This in turn, may lead to the loss of OD-neuron connections and axon degeneration. Myelin basic protein (MBP), myelin CNPase, and CD140a are specific markers that allow identification of oligodendrocytes.
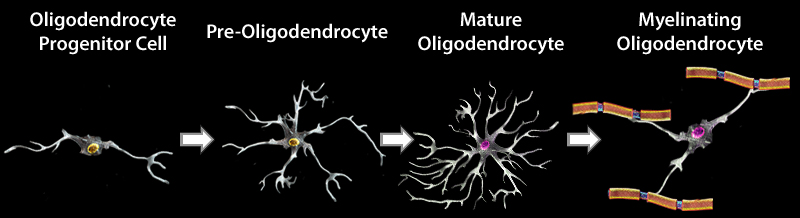
OD development and maturation can be divided into four stages in mice. During embryonic development, neural stem cells (NSCs) give rise to oligodendrocyte precursor cells (OPCs). OPCs then proliferate and migrate to different regions of the brain, and subsequently differentiate into pre-oligodendrocytes (pre-OLs). These cells can further mature into myelinating oligodendrocytes. OPCs continue to proliferate and differentiate into ODs in the adult brain. Each stage in the development and maturation of ODs can be identified using a panel of markers.
Oligodendrocytes
| A2B5 | A2B5 | Adenomatous polyposis coli (APC) | CNP |
| CNP | CNP | CNP | MAG |
| NG2 | NG2 | GalC | MOG |
| Nkx2.2 | Nkx2.2 | MBP | O4 |
| Olig1/2 | O4 | O4 | Olig1/2 |
| Sox-10 | Olig1/2 | Olig1/2 | Sox-10 |
| PDGFRα (CD140a) | Sox-10 | Sox-10 | |
| Vimentin | PDGFRα (CD140a) | ||
| Vimentin |
Schematic representation demonstrating morphological alteration and antigenic marker expression in each developmental stage in OD maturation.
References
- Zuchero JB and Barres BA. 2013. Curr. Opin. Neurobiol. 23:914. PubMed
- Mitew S, et al. 2014. Neuroscience. 12:29. PubMed
- Traiffort E, et al. 2016. J. Dev. Biol. 4 :28. PubMed
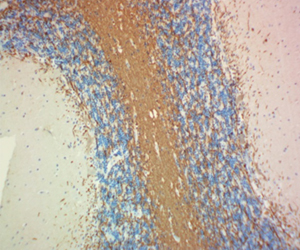
CNPase is a myelin-associated enzyme that is highly expressed in oligodendrocytes (ODs) and its appearance correlates with early events in OD differentiation. In the brain, CNPase associates with tubulin heterodimers and catalyzes microtubule formation. Due to its association with ODs and myelin production, this enzyme is of considerable interest in disorders affecting myelin such as multiple sclerosis (MS). BioLegend's clone SMI 91 is used for visualization of mammalian Myelin CNPase. This clone is effective for use in IHC, IF, and WB.
FFPE human brain tissue stained with anti-Myelin CNPase antibody (clone SMI 91).
Myelin Basic Protein
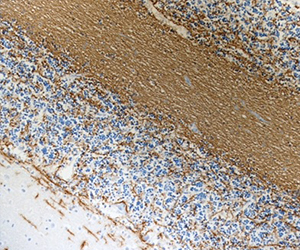
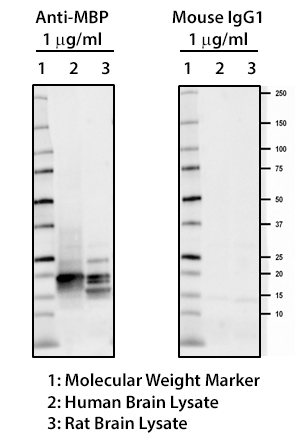
MBP plays an important role in the process of myelination by interacting with the lipids of the myelin membrane and maintaining the correct structure of myelin. Mice deficient in MBP demonstrate decreased CNS myelination leading to progressive disorders characterized by seizures and tremors. Autoreactive antibodies against MBP have been shown to contribute to the pathogenesis of MS, highlighting the importance of this target in the CNS. BioLegend offers several monoclonal antibodies (P82H9, SMI 94, SMI 99) for detection of MBP. These clones, along with clone SMI 91, are effective for IHC and WB. They are particularly useful for IHC studies on the progression of normal and pathological myelination and OD development.
Myelin Protein Zero
Myelin protein zero (MPZ), is a membrane glycoprotein mainly expressed in the peripheral nervous system (PNS). It is the major structural component of the myelin sheath, and accounts for at least 50% of all proteins in the PNS. MPZ is expressed by Schewann cells. Mutations in MPZ have been associated with myelin deficiency, which may lead to neuropathies including charcot-Marie-Tooth and Dejerine-Sottas diseases. BioLegend's Clone 58A is used for visualization and biochemical assessment of mammalian MPZ. This clone is effective for use in IHC staining and WB.
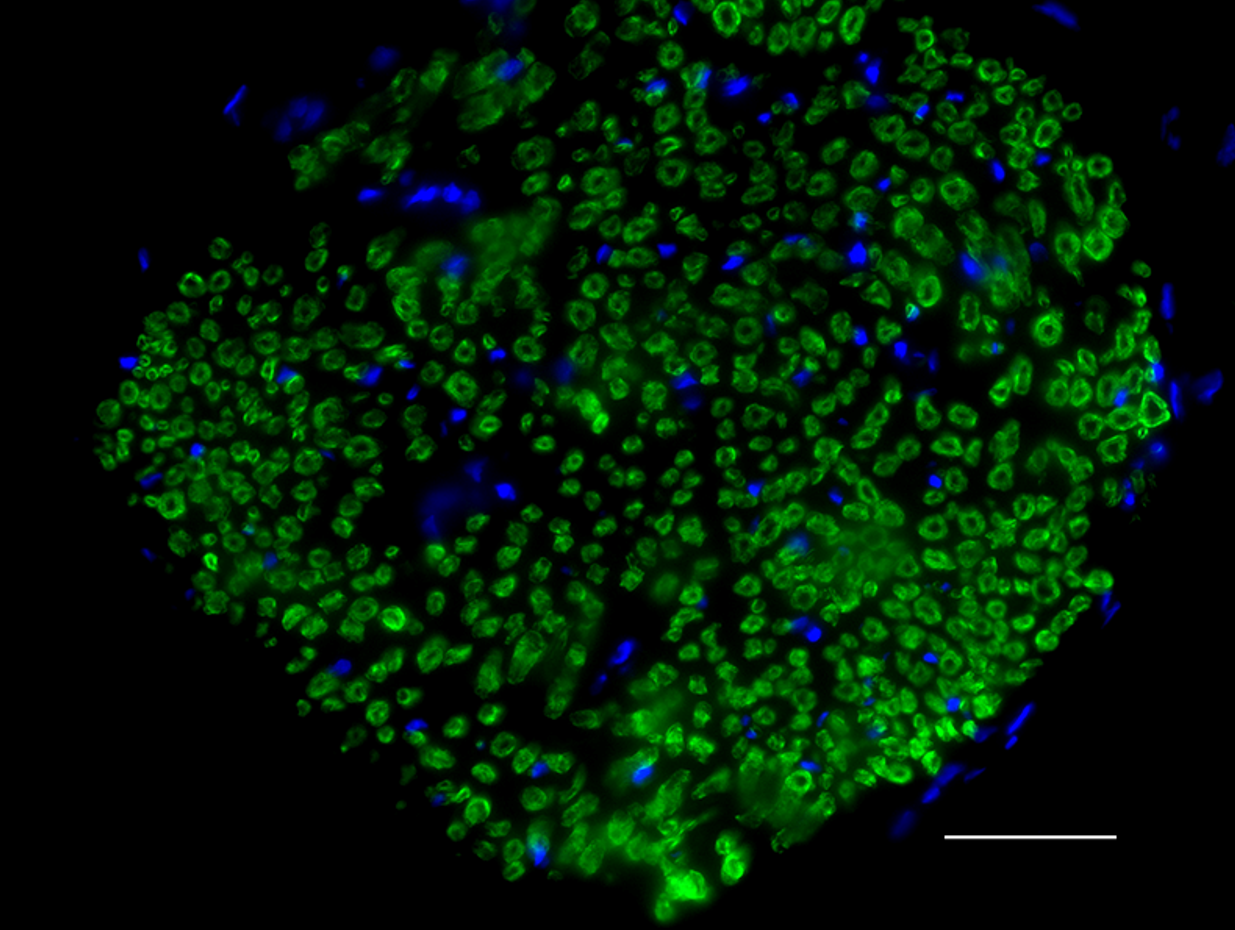
FFPE rat sciatic nerve tissue stained with Alexa Fluor® 488 anti-Myelin Protein Zero antibody (clone 58A).
Demyelination in Multiple Sclerosis (MS)
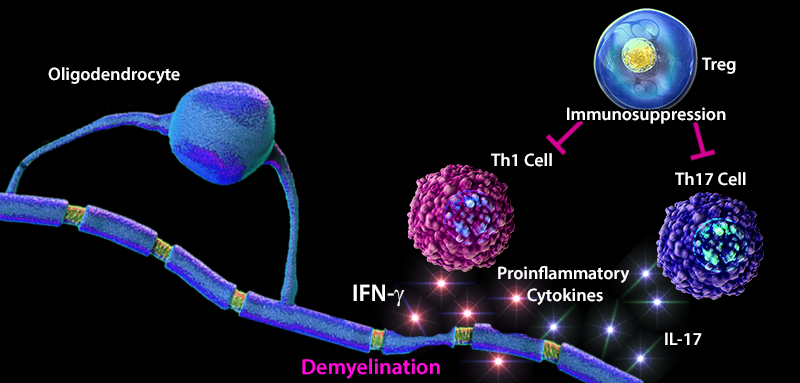
Multiple sclerosis (MS) is an inflammatory disease that is caused by the destruction of myelin sheaths and demyelination of axons. Maintaining the balance between effector and regulatory T cells (Tregs) plays an important role in the development of MS. An imbalance favoring effector T cells can often lead to a hyperactive immune system resulting in inflammation, tissue damage, and destruction. In MS patients, an increase in the number of effector Th1 and Th17 cells, and elevated levels of pro-inflammatory cytokines (e.g. IL-1, IL-6, TNF-α) have been reported. Furthermore, lower thymic production of Treg cells and a decreased diversity in the T cell receptor (TCR) repertoire attributed to defects in transforming growth factor beta (TGF-β) signaling has been observed in MS patients (1). TGF-β, a highly expressed cytokine in the CNS, has been shown to positively regulate the differentiation of naïve CD4+ T cells into CD4+ CD25+FOXP3+ Treg cells, and lead to adequate recognition and suppression of immune responses to self-proteins such as myelin. In contrast to myelin sheath damage caused by CNS infiltrating effector T cells, Treg cells demonstrate regenerative properties, and have emerged as mediators of oligodendrocyte differentiation and (re)myelination, thus promoting tissue regeneration and resolution of inflammation (2). These findings highlight the cooperative function of signaling molecules and immune cells in regulating the immune response and prevention of disease development.
References:
1. Lee PW, et al. 2017. Eur. J. Immunol. 47:446. Pubmed
2. Dombrowski Y, et al. 2017. Nat Neurosci. 20:674. Pubmed
 Login / Register
Login / Register 






Follow Us