The matrix effect in immunoassays: avoiding it in your ELISA and multiplex assays
The use of immunoassays for the quantification of analytes in biological samples such as, plasma, serum, tissue homogenates, etc. can be challenging. There are many components in these samples, such as carbohydrates, proteins, and phospholipids that can interfere with the ability of the antibody pairs to bind to their target. This phenomenon is known as the "Matrix Effect".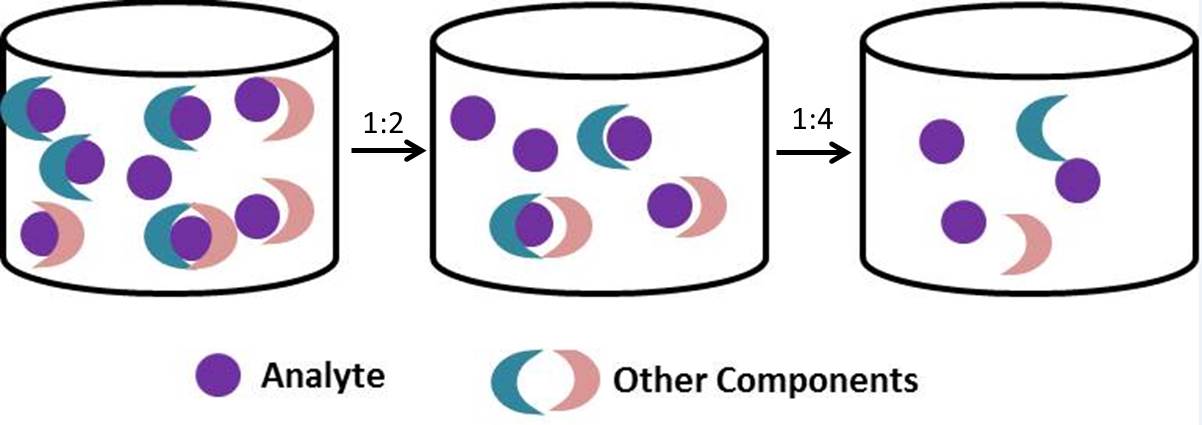 |
 The Matrix. Village Roadshow Pictures. |
| How do you know that you have entered the "Matrix"? |
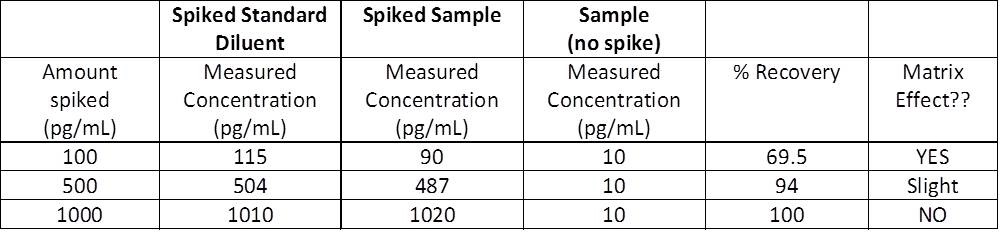
| Usually, the most obvious way to suspect you may have an issue due to the matrix effect, is to obtain OD readings much lower than expected. However, be aware that low OD readings can be due to other factors, such as low concentration of analyte in the sample, too short of an incubation time, etc. In order to determine if you are indeed encountering a problem with the matrix, there is something that you can do: perform a spike and recovery assay. Spike and recovery measurements will allow you to assess if your sample matrix (plasma, serum, etc.) is causing interference with the ability of the capture and detection antibodies to bind to the target protein being assayed. By adding, or "spiking", a known concentration of the recombinant standard into your sample and comparing this to the same concentration of recombinant spiked into the standard diluent, or blank, you can assess whether anything in your sample matrix is causing interference. You should always include your sample with no spiked recombinant so you are able to measure any endogenous protein that may already be there. All three of these samples are measured and concentrations are determined relative to the standard curve. In a perfect world, your recovery would be 100%. Ideally, you should shoot for somewhere between 80-120%. |
| What does that all mean and how do I determine the percent recovery? |
A simple equation...of course... |
 |
|
| Now what? You've determined the matrix effect is your problem... how do you overcome it? |
There are a couple of ways you can do this:
|
 |
| How do you know that you have entered the "Matrix"? |
| BioLegend has included specially formulated 'Matrix Diluents' in many of their ELISA and LEGENDplex™ Multiplex Assays in order to counteract the matrix effect. So, if you have ever asked yourself why your ELISA contains a 'Matrix Diluent' in addition to the Assay Diluent, and what exactly is it for... now you know. It's there to make sure you overcome the Matrix Effect. |
| A common question asked... If I am diluting my standards and samples with the Matrix Diluent, do I need to account for that dilution factor when analyzing my data? |
| You will notice that some of our ELISA or LEGENDplex™ kits ask you to dilute your standards in matrix buffers when working with serum or plasma samples in order to deal with the matrix effect. The final values that you report for your assay depend on what you want to calculate a concentration for - do you want to calculate the concentration in the actual sample or the total concentration in the well? There are two different scenarios... |
| Scenario A: This is how BioLegend performs the calculations. If you are looking to only measure the concentration in your actual sample, you will keep the concentrations listed in the manual for the standard curve. There will be no need to account for the 2-fold dilution factor, because both the standards AND samples have been diluted 1:1. However, if you diluted your samples PRIOR to this, this dilution factor would need to be accounted for. |
 Saturday Night Live. NBC |
| Manual Instructions: Top Standard Concentration: 1000 pg/mL, 50μL standards + 50μL samples per well. | 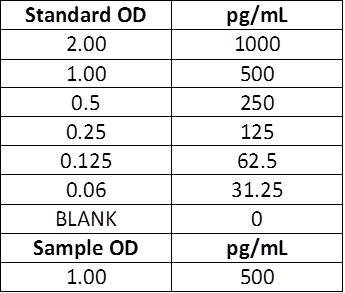 |
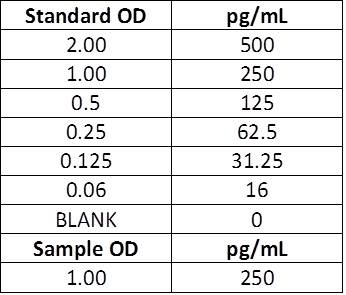
| Scenario B: If you prefer to measure the total concentration in the well, the standard values will need to be halved. After running the ELISA, the concentrations measured are the total concentrations in that well. To determine the concentration that is actually in your sample, you need to account for the 2-fold dilution. Any prior dilutions, as mentioned above, will also need to be accounted for. |
| Manual Instructions: Top Standard Concentration: 500 pg/mL, 50μL standards + 50μL samples per well Actual sample concentration: Calculated concentration (250 pg/mL) x Dilution factor (2) = Actual concentration (500 pg/mL) |
 |
| By following these guidelines, you will be able to determine whether you are running into any issues with regards to your samples and the matrix effect and learn how to take steps to correct that. For more information on the types of immunoassays that BioLegend sells, don't forget to check out our ELISA Kits and Sets and LEGENDplex™ Multiplex Assays. If you have any additional questions regarding the matrix effect or ELISA troubleshooting in general, check out our ELISA Troubleshooting Guide or contact our Technical Services Team at tech@biolegend.com. |
| Contributed by Kellie Johnson. |
 Login / Register
Login / Register 






Follow Us