The initiation and control of lymphocyte responses depend on the interaction of T cells with dendritic cells (DCs), a key type of antigen presenting cells (APCs). DCs are a complex innate immune cell population that recognize and respond to pathogen-associated and danger-associated signals. DCs are derived from bone-marrow progenitors and can be found in blood, lymphoid organs, and various tissues. Upon capture and process of pathogen-derived material, DCs present antigens in the context of MHC molecules on the surface of the cell. The interaction between TCR/co-receptor and MHC/peptide complex, and other co-stimulatory molecules presented on the surface of T cells and DCs, respectively, leads to activation and differentiation of naïve T cells.
Dendritic Cells
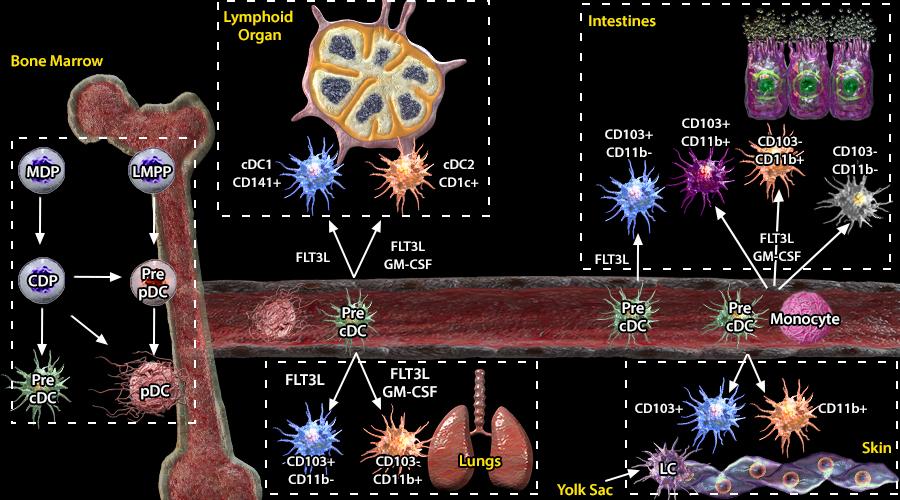
Plasmacytoid DCs (pDCs) originate from myeloid (MDP) and lymphoid progenitors (LMPP) in bone marrow. This process includes sequential differentiation of MDP and LMPP to common dendritic cell precursors (CDP; mouse and human) and pre-pDC (mouse), respectively (1,2,5,6). The direct precursor of mouse and human classical/conventional DCs (cDCs), pre-cDC, is also derived from CDP. In comparison to pre-pDC, pre-cDCs undergo DC differentiation upon migration to tissues (3,4). In mice, several pre-cDC subsets have been identified (Siglec-H+Ly6C-, Siglec-H+Ly6C+, Siglec-H-Ly6C+, and Siglec-H-Ly6C-). Siglec-H and Ly6C serve as lineage markers that distinguish pre-cDC subpopulations committed to the cDC1 (Siglec-H-Ly6C- pre-DCs) or cDC2 lineages (Siglec-H-Ly6C+ pre-DCs)(1).
DC differentiation and maintenance strongly depends on the presence of FLT3L cytokine. In addition, cDC2 differentiation and CD11b+CD103- and CD11b-CD103- cell populations in the gut can be driven by monocytes in the presence of another cytokine, GM-CSF (7). In contrast, Langerhans cells are not derived from bone marrow progenitors. Instead, they differentiate early in ontogenesis from an embryonic cell progenitor present in the yolk sac (7).
| MDP: Monocyte/DC Progenitor LMPP: Lymphoid Primed Multipotent Progenitor Pre-cDC: Precursor of conventional/classic DC Pre-pDC: Precursor of plasmacytoid DC (in mice) CDP: Common Dendritic Cell Progenitor pDC: Plasmacytoid DC LC: Langerhans Cell |
cDC1: Conventional/Classical Dendritic Cell 1 or CD8a+ DC. Human CD141 DCs are an equivalent of cDC1 cells in mice. cDC2: Conventional/Classical Dendritic Cell 2 or CD11b+ DC. Human CD1c DCs are an equivalent of cDC2 cells in mice. |
References:
- Schlitzer A., et al. 2015. Nat. Immunol. 16:718. PubMed
- Onai N., et al. 2013. Immunity. 38:943. PubMed
- Breton G., et al. 2015. J. Exp. Med. 212:401. PubMed
- Naik S. H., et al. 2006. Nat. Immunol. 7:663. PubMed
- Liu K., et al. 2009. Science. 324:392. PubMed
- Lee J., et al. 2017. Nat. Immunol. 18:877. PubMed
- Bekiaris V., et al. 2014. Immunol. Rev. 260:86. PubMed
Technical advances, such as multiparameter flow cytometry and genetic tools including single-cell transcriptomics, have led to the realization that DCs are a diverse population of cells from a developmental and functional standpoint. Based on function, phenotype, and tissue distribution, DCs can be divided into two major subsets: plasmacytoid dendritic cells (pDCs) and conventional dendritic cells (cDCs). cDCs are found across lymphoid organs (thymus, spleen, and LN) and non-lymphoid organs (lung, gut, and skin). In mice, cDCs populating the lymphoid organs are subdivided into cDC1 and cDC2 cells, while the cDCs in non-lymphoid organs are subdivided into CD103+ and CD11b+ cells. Similarly, human cDCs are subdivided into CD141+/BDCA3+ and CD1c+/BDCA1+ cells in spleen and lung, CD103+ and CD103- cells in the intestine, and CD14+ and CD14- cells in the dermis. Besides pDCs and cDCs, there are specialized antigen presenting cells called Langerhans cells (LC), which can be found selectively in the epidermis.
| Markers | Lymphoid Tissue Resident DCs | Primary Non-Lymphoid Tissue Resident DCs | ||||||||
| Spleen, LN, Thymus (1,9) | Lung, Intestine (1,2,3,4,8,9) | Intestine (1,3,4,8,9) | Skin (1,5,6,9) | |||||||
| Epidermis | Dermis | |||||||||
| pDC | cDC1/ CD8a+ | cDC2/ CD11b+ | CD103+ CD11b- | CD103-CD11b+ | CD103+ CD11b+ |
CD103- CD11b- |
LC | CD103+ DDC | CD11b+ DDC | |
| Linⱡ | + | + | + | |||||||
| CD45 | + | + | + | + | + | + | + | + | + | + |
| CD11c | + | ++ | ++ | ++ | ++ | ++ | ++ | + | + | + |
| MHC II | lo/- | ++ | ++ | ++ | ++ | ++ | ++ | + | + | + |
| Siglec H* | + | - | - | - | - | - | - | - | - | - |
| B220* | + | - | - | - | - | - | - | - | - | - |
| BST2*/CD317 | + | - | - | - | - | - | - | - | - | - |
| CD8a* | - | + | - | - | - | - | - | |||
| CLEC9A*/DNGR1 | + | ++ | - | ++ | - | - | - | + | - | |
| CD24* | - | + | lo/- | + | lo/- | + | + | + | ||
| CD11b* | - | lo/- | + | - | + | + | - | + | - | ++ |
| SIRP1α/CD172* | lo/- | + | - | + | + | - | + | + | ||
| CD103 | - | subset | - | ++ | - | ++ | - | - | + | |
| CD135/FLT3 | + | + | +/- | - | ||||||
| CX3CR1 | + | + | + | |||||||
| CD205 | - | + | lo | + | + | + | ||||
| CD209a/ DC-SIGN |
+ | - | + | - | +/- | + | ||||
| Langerin/CD207 | - | + | - | + | + | ++ | + | - | ||
| E-Cadherin | ++ | |||||||||
| Ep-CAM | - | - | - | - | - | - | + | lo/- | ||
| XCR1 | - | + | - | + | - | - | - | + | - | |
| CD64 | - | - | - | - | - | - | - | |||
| F4/80 | - | - | lo/- | - | lo/- | - | + | |||
| CD80 | + | + | + | + | + | + | + | + | + | + |
| CD86 | + | + | + | + | + | + | + | + | + | + |
| ¶Human DC cell equivalent | pDC | CD141+/ BDCA3+ | CD1c+/ BDCA1+ | CD141+/ BDCA3+ or CD103+ XCR1+ |
CD1c+/ BDCA1+ or CD103- XCR1- |
CD103+ XCR1- | ? | LC | CD14- DDC | CD14+ DDC |
| pDC: Plasmacytoid Dendritic Cell cDC1/CD8a+: Conventional/Classical Dendritic Cell 1 or CD8a+DC cDC2/CD11b+: Conventional/classical Dendritic Cell 2 or CD11b+DC LC: Langerhans Cell DDC: Dermal Dendritic Cell |
|
| ⱡLin (CD3-, Ly6G-, CD19-, NK1.1/CD49b-) *Siglec H, B220, and BST-2 markers can be used interchangeably to identify pDCs. Similarly, CD8a, CLEC9A and CD24 markers are used interchangeably to identify cDC1, and CD11b and SIRP1α/CD172a are used interchangeably to identify cDC2. ¶Human equivalents of the mouse lung and intestinal CD103+CD11b- cells are CD141+/BDCA3+ or CD103+/XCR1+ subsets. Similarly, the analog of the mouse lung and intestine CD103-CD11b+ cells are CD1c+/BDCA+ or CD103-/XCR1- cells. Learn more with Cell Marker and Maturation Marker Webpages References:
|
Technical advances, such as multiparameter flow cytometry and genetic tools including single-cell transcriptomics, have led to the realization that DCs are a diverse population of cells from a developmental and functional standpoint. Based on function, phenotype, and tissue distribution, DCs can be divided into two major subsets: plasmacytoid dendritic cells (pDCs) and conventional dendritic cells (cDCs). cDCs are found across lymphoid organs (thymus, spleen, and LN) and non-lymphoid organs (lung, gut, and skin). In mice, cDCs populating the lymphoid organs are subdivided into cDC1 and cDC2 cells, while the cDCs in non-lymphoid organs are subdivided into CD103+ and CD11b+ cells. Similarly, human cDCs are subdivided into CD141+/BDCA3+ and CD1c+/BDCA1+ cells in spleen and lung, CD103+ and CD103- cells in the intestine, and CD14+ and CD14- cells in the dermis. Besides pDCs and cDCs, there is specialized antigen presenting cells called Langerhans cells (LC), which can be found selectively in the epidermis.
| Markers | Lymphoid Tissue Resident DCs | Primary Non-Lymphoid Tissue Resident DCs | |||||||||
| Spleen (1,2,8) | Lung, (3,4) | Intestine (5) | Skin (7) | ||||||||
| Epidermis | Dermis | ||||||||||
| pDC | CD141+/ BDCA3+ | CD1c+/ BDCA1+ | CD141+/ BDCA3+ | CD1c+/ BDCA1+ | CD103+ XCR1+ | CD103-XCR1- | CD103+ XCR1- | LC | CD14- DDC | CD14+ DDC | |
| Linⱡ | + | + | + | ||||||||
| CD45 | + | + | + | + | + | + | + | + | + | + | + |
| CD11c | lo/- | + | + | + | + | ||||||
| HLA-DR | + | + | + | + | + | + | + | + | + | + | + |
| CD303*/ BDCA-2 |
+ | - | - | ||||||||
| CD45RA* | ++ | - | - | ||||||||
| CD123* | ++ | lo | lo | ||||||||
| CD141*/BDCA-3 | ++ | - | ++ | - | + | - | - | ||||
| CLEC9A*/DNGR1 | ++ | - | ++ | - | + | - | - | ||||
| CD1c*/BDCA-1 | - | + | - | + | + | + | |||||
| SIRP1α*/CD172 | - | + | - | + | - | + | + | ||||
| CD11b | + | + | |||||||||
| FLT3 | + | + | + | ||||||||
| CD1a | + | +/- | |||||||||
| CD209/DC-SIGN | + | ||||||||||
| Langerin/CD207 | + | ||||||||||
| E-Cadherin | + | ||||||||||
| EpCAM | + | ||||||||||
| CD103 | + | - | + | ||||||||
| XCR1 | + | - | - | ||||||||
| CD14 | - | + | |||||||||
| CD116/ GM-CSFRα |
lo | + | lo | ||||||||
| CD80 | + | + | + | + | + | + | + | + | + | + | + |
| CD86 | + | + | + | + | + | + | + | + | + | + | + |
| Mouse DC equivalent | pDC | cDC1/ CD8a+ | cDC2/ CD11b+ | CD103+ CD11b- | CD103-CD11b+ | CD103+ CD11b- | CD103-CD11b+ | CD103+ CD11b+ | LC | CD103+ DDC | CD11b+ DDC |
| pDC: Plasmacytoid Dendritic Cell LC: Langerhans Cell DDC: Dermal Dendritic Cell cDC1/CD8a+: Conventional/Classical Dendritic Cell 1 or CD8a+DC cDC2/CD11b+: Conventional/Classical Dendritic Cell 2 or CD11b+DC |
|
| ⱡLin (CD3-, CD20-, CD66b-, CD335-) *CD45RA and CD303 markers are used interchangeably to identify pDCs. Similarly, CLEC9A and CD141 markers are used interchangeably to identify CD141+/BDCA3+ cDC, and CD1c and SIRP1α markers are used interchangeably to identify CD1c+/BDCA1+cDC. Learn more with Cell Marker and Maturation Marker Webpages References:
|
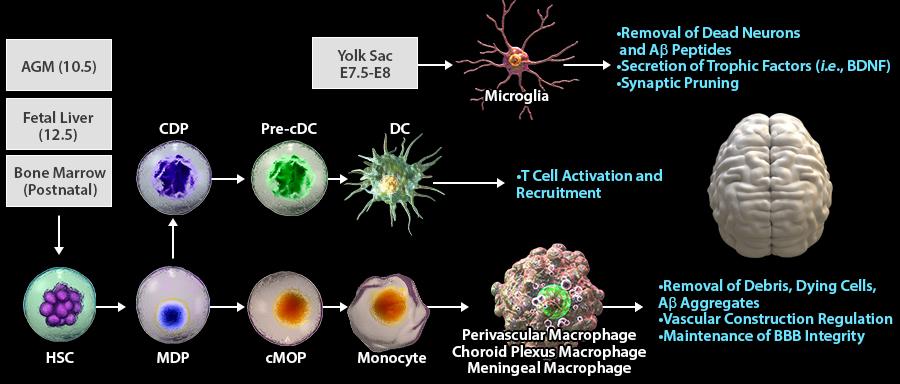
In the steady state, the brain contains several populations of myeloid cells located at distinct sites. Microglia cells are identified in the parenchyma, while macrophages and some dendritic cells can be found in the perivascular region, choroid plexus, and meninges. Each of these cell types is derived at a different time in ontogenesis and execute various tasks during homeostasis and surveillance. Microglia cells are derived from erythromyeloid progenitors in the yolk sac during primitive hematopoiesis happening around embryonic day 7.5-8 (E7.5-8). CNS macrophages are differentiated from different sources during embryogenesis, first in the aorta–gonad–mesonephros (AGM) region at E10.5 and then in the fetal liver (E12.5) during definitive hematopoiesis. Postnatally, the primary source of macrophages are monocytes differentiated from bone marrow HSC progenitors through subsequent differentiation stages including MDP and cMOP. DCs are also derived from MDP through subsequent differentiation of MDP to CDP and pre-cDC.
In homeostasis, the neuro-supportive role of microglia is mediated by the secretion of trophic factors, such as BDNF (brain-derived neurotrophic factor), pruning of neuronal synapses and removal of dead cells and β-amyloid peptides. Macrophages play an essential role in the uptake of local debris and dying cells. Perivascular macrophages clear β-amyloid from CNS and maintain brain blood-barrier integrity through regulation of vascular construction.
Under acute (e.g., ischemic stroke) or chronic inflammatory signals (e.g., Parkinson's disease or Alzheimer’s disease), microglial cells become activated and differentiate into effective APCs. The inflammatory response in the brain attracts peripheral DCs to the inflammation site for antigen uptake. Upon antigen uptake and maturation, DCs migrate to secondary lymphoid organs to present these antigens to T-cells in order to activate neuroprotective or neurodestructive pathways mediated by Tregs or Th1/Th17 cells, respectively.
HSC: Hematopoietic Stem Cell
MDP: Monocyte/DC Progenitor
CDP: Common Dendritic Cell Progenitor
Pre-cDC: Precursor of conventional/classic DC
cMOP: Common Monocyte Progenitor
DC: Dendritic Cell
| Markers downregulated upon activation |
| Markers upregulated upon activation |
Learn more about Microglia function in different diseases with our Microglia webpage.
References:
- Prinz M., et al. 2011. Nat. Neurosci. 14:1227. PubMed
- Prinz M. and Priller J. 2014. Nat. Rev. Neurosci. 15:300. PubMed
- Ludewig P., et al. 2016. Biochim. Biophys. Acta. 1862:352. PubMed
- Herz J., et al. 2017. Immunity. 46:943. PubMed
BioLegend offers a comprehensive portfolio of products to study macrophages and DCs, and interrogate their involvement in inflammatory pathways in the CNS.
Alexa Fluor® 488 anti-mouse F4/80 Antibody (clone BM8)
Clone BM8 is used for visualization of murine tissue macrophages. This clone is effective for use in IHC and FC.
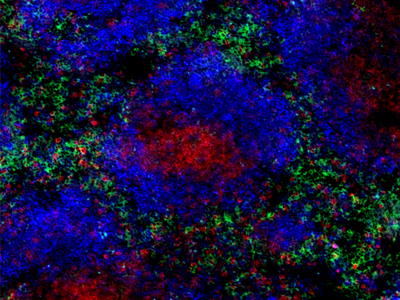
Frozen mouse spleen section co-stained with Alexa Fluor® 488 anti-mouse F4/80 (clone BM8, green), Brilliant Violet 510™ anti-mouse/human CD45R/B220 (clone RA3-6B2, blue) and Brilliant Violet 421™ anti-mouse CD3 (clone 17A2, red) antibodies.
Purified anti-CX3CR1 Antibody (clone 8E10.D9)
Clone 8E10.D9 demonstrates specificity for human CX3CR1 and is effective for use in IHC and WB. This clone is available in a purified and biotin-conjugated formats.
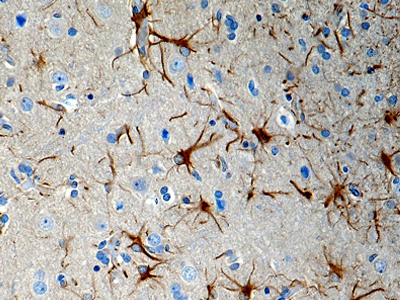
FFPE human brain tissue stained with anti-CX3CR1 antibody (clone 8E10.D9).
Brilliant Violet 510™ anti-human CD11c Antibody (clone 3.9)
Clone 3.9 preferentially binds the activated form of CD11c and is specific for the I domain of CD11c. CD11c, in combination with other markers (e.g. CD141), is used for visualization of human dendritic cells. This clone is effective for use in IHC and FC.
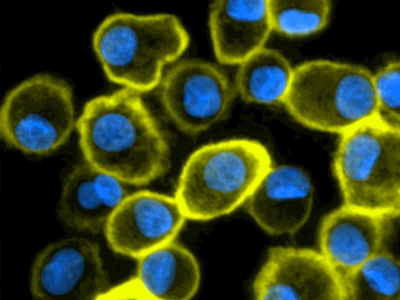
Human peripheral blood mononuclear cell (PBMC)-derived dendritic cells stained with Brilliant Violet 510™ anti-human CD11c antibody (clone 3.9, yellow). Nuclei were counterstained with DAPI (blue).
Purified anti-P2RY12 Antibody (clone S16007D)
Clone S16007D demonstrates specificity for murine P2RY12 and is effective for use in IHC, IF and FC.
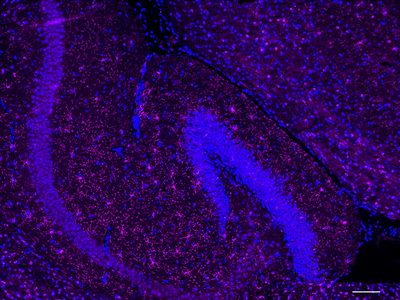
IHC staining of purified anti-P2RY12 antibody (clone S16007D) on formalin-fixed paraffin-embedded mouse brain tissue.
Brilliant Violet 711™ anti-human CD163 Antibody (clone GHI/61)
Clone GHI/61 binds to domain 7 of CD163. CD163 can be used as a marker for macrophages in human tissues. This clone is effective for use in FC.
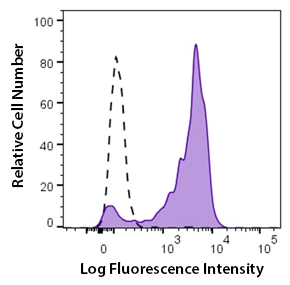
Human peripheral blood monocytes stained with Brilliant Violet 711™ CD163 antibody (clone GHI/63, filled histogram) or Brilliant Violet 711™ mouse IgG1 isotype control (open histogram).
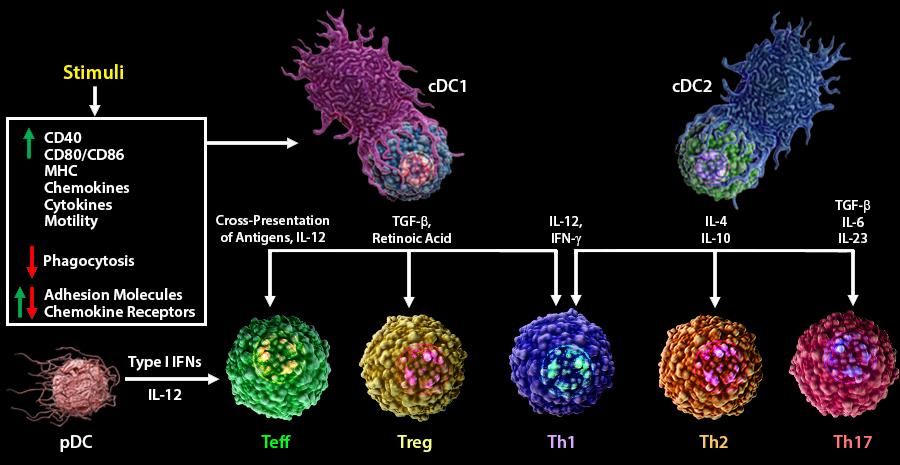
Upon pathogen encounter, DC cells undergo a process of morphological and functional changes known as maturation. This process includes:
Phenotypic changes: Up-regulation of MHC and co-stimulatory molecules and changes in the pattern of expression of adhesion molecules and chemokine receptors.
Morphological changes: Development of dendrites and cytoskeleton reorganization.
Functional changes: Down-regulation of antigen uptake and secretion of chemokines and cytokines.
Upon maturation, DC cells migrate to lymphoid organs where they interact with naïve T cells to drive their activation and differentiation to cytotoxic and helper subsets.
Learn More With Webpages And Interactive Pathway Posters:
Nature Reviews Poster: Dendritic Cells: Controllers of Adaptive Immunity
Dendritic Cell, Monocyte, and Macrophage Biology Poster
Innate Immune Signaling Poster
Innate Immune Signaling Webpage
Toll-Like Receptors Webpage
References:
- Pakalniškytė D. and Schraml B.U. 2017. Adv. Immunol. 134:89. PubMed
BioLegend offers a wide range of high quality and specific products to study dendritic cells. These reagents include antibodies, functional recombinant proteins, immunoassays, and magnetic cell separation kits that enable research into dendritic cell heterogeneity and function utilizing multiple applications.
Antibodies
Human Dendritic Cell Antibodies
Mouse Dendritic Cell Antibodies
Recombinant Proteins
Featured products for DC cell isolation
MojoSort™ Mouse Pan Dendritic Cell Isolation Kit
MojoSort™ Mouse CD11c Nanobeads
This magnetic bead-based cell separation kit allows isolation of mouse dendritic cells for downstream applications downstream applications include functional assays, gene expression, phenotypic characterization, etc. Alternatively, you can use our nanobeads for positive selection of CD11c+ mouse cells.
Featured products for DC cell differentiation in vitro and in vivo
Recombinant Human FLT3L (carrier-free)
Recombinant GM-CSF (carrier-free)
LEGENDplex™ HSC Myeloid Panels
Featured products for DC cell functional analysis
LEGENDplex™ inflammation panels
Proteogenomics

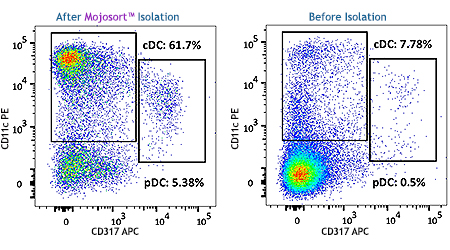
A single cell suspension from C57BL/6 mouse splenocytes was prepared using enzymatic digestion to isolate Pan Dendritic Cells (classical and plasmacytoid) using the MojoSort™ Mouse Pan Dendritic Cell Isolation Kit. Cells were stained with anti-mouse CD11c (clone N418) PE and anti-mouse CD317 (clone 927) APC. Dead cells were excluded by Helix NP™ Blue.
 Login/Register
Login/Register 






Follow Us