Alzheimer's disease
| This is a topic very close to my heart. My grandmother had Alzheimer's disease (AD). I have very fond memories of visiting her as a kid and enjoying every bit of her love and pampering, and her grandmotherly concerns and protectiveness. She had seven grandsons and I was her only granddaughter. So I was very special to her, as she was to me. As I was growing up, we noticed that she was starting to forget things. In the beginning, they were just little things, like not being able to remember where she kept her belongings. But slowly, over the years, they started getting more and more serious, until one day, when she called me by my mother's name and asked me to sit next to her. Then, not recognizing who I was, she started talking to me, confusing me for my mother! That day, for the first time, I realized that things were never going to be the same again. |
|
| AD is a neurodegenerative disease that is the most common cause of dementia among older people. In 2014, nearly 36 million people were diagnosed with this disease worldwide. Symptoms of AD include loss of cognitive function and behavioral abilities, usually to such an extent that it interferes with a person's daily life and activities. Most people with AD suffer from the late-onset disease that usually develops after 60 years of age. Early-onset AD is a rare form of the disease which occurs in people between 30 to 60 years and represents less than 5 percent of all people who have AD. The disease progresses through several stages – from mild to moderate to severe. In the later stages, this disease is quite aggressive and causes death in 68% of all diagnosed cases. | |
| AD is named after the German psychiatrist Dr. Alois Alzheimer. In 1906, Dr. Alzheimer reported the first case of AD in one of his patients, a 55-year-old woman named Auguste Deter, who had died of an unusual mental illness. After the patient's death, he noticed changes in the brain tissue, including many abnormal clumps and tangled bundles of fibers, which are today identified as amyloid plaques and neurofibrillary tangles (NFT). Abnormal deposits of plaques and tangles in the brain are hallmark features of AD. Over time, these protein aggregations cause neuronal death and damage to the hippocampus, which is a part of the brain essential for forming memories. Subsequently, the brain tissue itself begins to shrink and by the final stages of the disease, the brain may have shrunk dramatically. | |
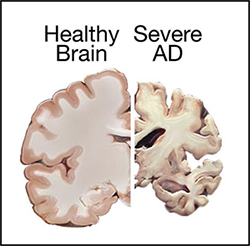 Comparison of a healthy brain and the brain of a patient with severe AD. |
|
|
Sadly, scientists don't yet fully understand what causes AD. Various inflammatory processes and cytokines are thought to play a role in the pathology of AD. Genetic mutations have also been observed in 1-5% of cases. Some of these mutations are in the genes encoding amyloid precursor protein (APP) (1). APP is a single-pass transmembrane protein with a large extracellular domain concentrated in the synapses of neurons. The physiological function of APP is largely undetermined. APP is cleaved within the transmembrane domain by γ-secretase (a protease complex) to produce amyloid-beta (Aβ) (2). The oligomerization of the Aβ peptides is the primary component of senile plaques found in the brains of AD patients (3). These plaques are known to cause cellular toxicity including disrupting the cell's calcium ion homeostasis, inhibiting utilization of glucose by neurons, inducing inflammation, oxidative stress and ultimately inducing programmed cell death (apoptosis) (4). |
|
| The other predominant contributor to AD is Tau hyperphosphorylation. Tau was first discovered in 1975 as a microtubule-associated protein (MAP) that stimulates tubulin assembly into axonal microtubules in the brain (5) There was not much research interest in Tau protein until a decade later, when it was found that abnormally hyperphosphorylated Tau make up the NFTs in an AD brain (6). Phosphorylation and dephosphorylation are part of the normal processing that Tau undergoes in the healthy neuron as a consequence of dynamic regulation of Tau kinases and phosphatases. However, the phosphorylation level of Tau isolated from an autopsied AD brain is 3- to 4-fold higher than that of normal human brains (7). Despite extensive studies, the causes leading to abnormal hyperphosphorylation of Tau are still not fully understood. | |
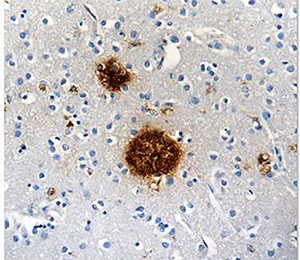 Protein aggregates, like amyloid beta shown here, can tip the scales in favor of AD. |
|
| The detection of AD is complicated. Although doctors now have several tools for AD diagnosis, such as CT scans and MRIs, this disease can only be definitively diagnosed after death, by examination of brain tissue. Also, there's little we can do to prevent AD and other types of dementia, making it very difficult for both the patient and the caregiver. Medication does not appear to be very useful as a treatment. Some lifestyle changes, such as modifying diet, or increasing intellectual activities, such as reading and playing musical instruments, might have some beneficial effects in reducing the risk for AD, but more clinical studies are required to confirm this. | |
| Scientists are also struggling to understand why this disease largely affects older adults. Increasing production of free radicals and shrinking of certain parts of the brain might contribute to this age-related disease. Unfortunately, very little is known about this disease, and so, extensive scientific studies are being conducted to understand what causes this disease and how to improve the current treatment options. To this extent, BioLegend now offers an entire catalog of products to support research efforts for these kinds of neurodegenerative diseases. We have new Tau products and APP/Aβ products. We also have a variety of other neuroscience-related products. Please let us know your thoughts or share any interesting facts you might know about this disease at tech@biolegend.com. | |
References:
|
|
| Contributed by Mohar Chattopadhyay, Ph.D. |
 Login / Register
Login / Register 






Follow Us