Deciphering immune cell responses to Long COVID and viral infections
In response to pathogens, especially viruses, cells release interferons and other cytokines to fight the infections. Interferons are typically divided into three types: I (e.g., IFN-α, IFN-β), II (e.g., IFN-γ), and III (e.g., IFN-λ1, IFN-λ2). All interferons have been shown to be important for fighting viral infections and for regulating the immune system. Interferons are also critically involved in cancer and autoimmune diseases such as psoriasis, systemic lupus erythematosus, and multiple sclerosis. Studying the expression profile of interferons and other related cytokines is pivotal to understanding the immune response against pathogens and related disease processes.
Long COVID, also known as post-acute sequelae of SARS-CoV-2 infection (PASC), refers to persistent symptoms that continue for weeks or months after the acute phase of COVID-19. Common symptoms include loss of taste or smell, fatigue or persistent tiredness, difficulty breathing even after recovery, persistent aches and discomfort in bone, chest, and joints, as well as cognitive difficulties, memory issues, and trouble concentrating.
Flow cytometry-based LEGENDplex™ assays can be used to assess cytokine production and immune activation in in vitro and biological samples caused by a novel biologic, therapeutic, or pathogen. Our panels can simultaneously detect multiple cytokines derived from monocyte, T cell, and NK cell activation and provide an in-depth analysis of the immune cell response following stimulation. Using our cytokine detection assays permit a broad assessment of cytokine production, which can help characterize immune cell responses in multiple research applications.
In a recent study, researchers used our reagents to characterize the immune response in patients experiencing Long COVID1. The authors mention that after acute SARS-CoV-2 infection, a subset of patients exhibited persistently high levels of interferon-γ (IFN-γ) from peripheral blood mononuclear cells (PBMCs).
Human IFN-γ is a potent multifunctional cytokine that is secreted primarily by activated NK cells and T cells. Originally characterized based on its anti-viral activities, IFN-γ also exerts anti-proliferative, immunoregulatory, and proinflammatory activities. IFN-γ can also upregulate MHC class I and II antigen expression by antigen-presenting cells.
Measuring cytokine secretion using LEGENDplex
Cytokine secretion between Long COVID and healthy control PBMCs were compared using LEGENDplex Human COVID-19 Cytokine Storm Panels 1 and 2. PBMCs samples from 10 healthy controls and 14 patients with Long COVID were analyzed. After 48 hours of incubation, medium was collected and loaded onto LEGENDplex plates, and cytokine concentrations were calculated and analyzed using our complimentary software. The authors determined that PBMCs from Long COVID spontaneously produced more pro-inflammatory cytokines.
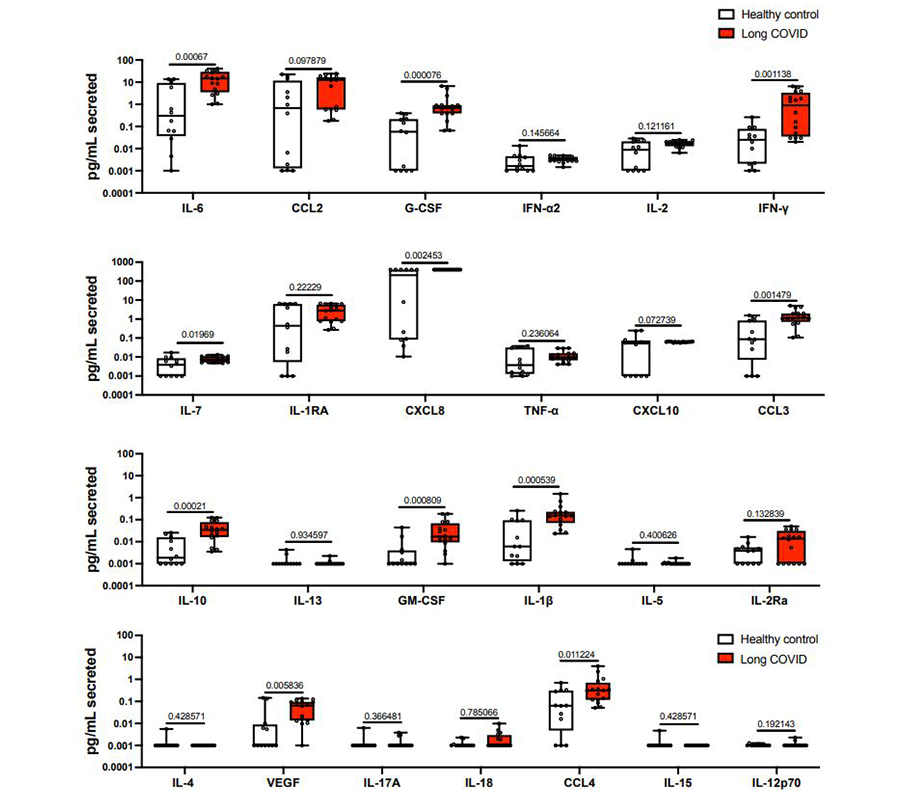
PBMCs from Long COVID spontaneously produce more pro-inflammatory cytokines1. PBMCs from patients with Long COVID (n=16) or healthy controls, either unexposed (n=7) or those recovered from acute COVID-19 (n=5), were incubated for 48 hours without exogenous peptide stimulation. Cytokine production was assessed using LEGENDplex. Creative Commons BY 4.0.
Furthermore, our ELISAs were used to confirm cytokine secretion. For these experiments, medium from PBMCs cultured for 48 hours without stimulation was used to measure the following cytokines using ELISA MAX™ Standard Sets for Human TNF-α, GM-CSF, as well as our ELISA MAX Deluxe Sets for Human IL-1β and IFN-γ.
Interestingly, unlike patients recovering from acute infection, those with Long COVID maintained elevated IFN-γ release even without ex vivo peptide stimulation. The IFN-γ release was further determined to be CD8+ T cell–mediated and dependent on antigen presentation by CD14+ cells. Targeting the IFN-γ pathway could be explored as a therapeutic approach, modulating this immune response might mitigate symptoms and improve patient outcomes.
Optimized immunoassays for human IFN-γ
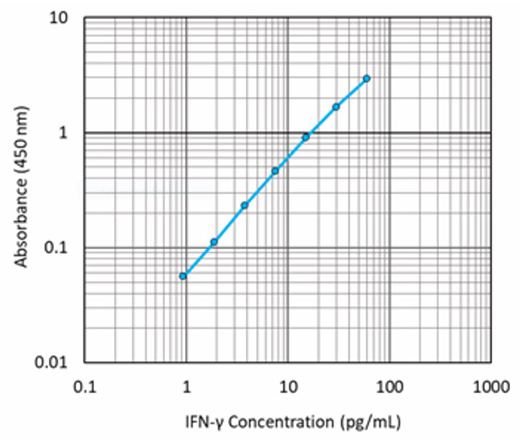
Representative sandwich immunoassay standard curve generated using serial dilutions of LEGEND MAX™ Human IFN-γ Standard ranging from 0.94 pg/mL to 60 pg/mL.
Our LEGEND MAX™ High Sensitivity Human IFN-γ ELISA Kit is analytically validated with ready-to-use reagents including, a 96-well strip plate that is pre-coated with a monoclonal anti-human IFN-γ antibody to detect low concentrations of IFN-γ. This kit is specifically designed for the accurate quantitation of human IFN-γ from serum, heparin plasma, and cell culture supernatant.
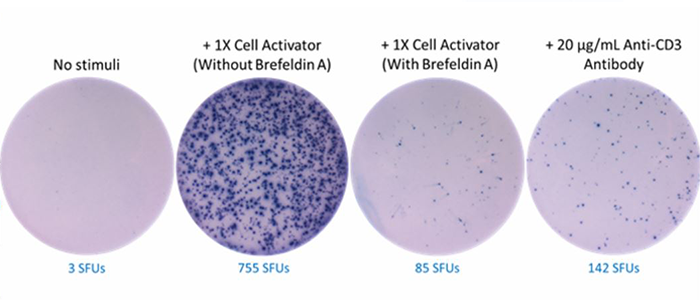
Human peripheral blood mononuclear cells at 104 cells/well were incubated for 20 hours in the absence or presence of 1X Cell Activator without Brefeldin A, 1X Cell Activator with Brefeldin A, or Ultra-LEAF™ purified anti-human CD3. Brefeldin A functions to inhibit intracellular protein transport. As a result, it inhibits secretion of cytokines such as IFN-γ. The number of cells secreting human IFN-γ was analyzed by ELISpot.
Featured LEGENDplex panel
Our Human Anti-Virus Response Panel V02 is a bead-based multiplex assay suitable for use on various flow cytometers that allows simultaneous quantification of 13 human proteins involved in the anti-virus immune response, including interferons (α, β, γ, λ1 and λ2), interleukins (1β, 6, 8, 10, 12), TNF-α, IP-10 and GM-CSF. The Type 1/2/3 Interferon Panel is a subpanel of the 13-plex Anti-Virus Response Panel, which allows for simultaneous quantification of the five interferons. Both panels provide high sensitivities and broad dynamic range, and have been validated for use on serum and cell culture supernatant samples.
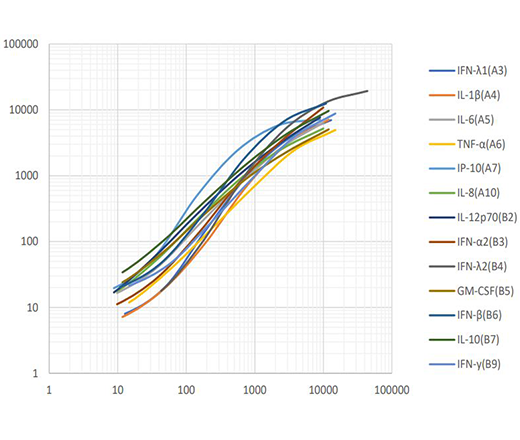
This standard curve was generated using the LEGENDplex Human Anti-Virus Response Panel for demonstration purpose only. A standard curve must be run with each assay.
Summary
These findings shed light on a potential mechanism underlying Long COVID, emphasizing the need for biomarkers and therapeutic approaches for this debilitating condition. Long COVID, characterized by relapsing and remitting symptoms, remains challenging to diagnose and treat, affecting patients regardless of the severity of their initial COVID-19 illness. Understanding the role of IFN-γ provides insights into the immune response to Long COVID as well as pathogenesis that could inform strategies for research and improve patient care2. Although further research is required to confirm IFN-γ as a potential biomarker, studies into this crucial pathway could aid our understanding and treatment of Long COVID. Monitoring IFN-γ levels over time may help track disease progression and response to treatment, as well as help guide immune profiling studies for viral infections.
Our reagents and solutions are designed for all aspects of your infectious disease research. Focus on single targets with our highly-sensitive ELISAs and ELISpot kits, or analyze multiple cytokines with our LEGENDplex multiplex immunoassays. For high throughput detection of cytokines, learn more about Revvity’s no-wash immunoassay technologies.
References
- Krishna, BA., et al. "Spontaneous, persistent, T cell–dependent IFN-γ release in patients who progress to Long Covid." (2024). Science Advances, vol. 10, no. 31. doi:10.1126/sciadv.adi9379. PubMed.
- M. Scherlinger, R., et al. “Refining “long-COVID” by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 infection.” (2021). Infect. Dis. Ther. 10, 1747– 1763. doi:10.1007/s40121-021-00484-w. PubMed.

 Login / Register
Login / Register 






Follow Us