BioLegend and MJFF
We partner with our customers and collaborators to create unique products that drive innovation and advance your research. Learn more about our commitment and partnership with The Michael J. Fox Foundation for Parkinson’s Research (MJFF) and our shared goal of advancing research in Parkinson’s disease.
A Strong Partnership with The Michael J. Fox Foundation for Parkinson’s Research
The Parkinson’s Progression Markers Initiative
The Parkinson’s Progression Markers Initiative (PPMI) is a collaboration of researchers, funders, and study participants working toward the goal of identifying progression biomarkers to improve PD therapeutics. PPMI is sponsored and partially funded by MJFF. Other partners include a consortium of industry players, non-profit organizations, and private individuals. We are proud to be a part of the Partner Scientific Advisory Board (PSAB) and to participate in the selection and review of potential progression markers that could be used in clinical testing.
MJFF is dedicated to finding a cure for Parkinson's disease and to ensuring the development of improved therapies for those living with Parkinson's today. MJFF engages partners to speed scientific breakthroughs in the search for a cure for Parkinson's disease. Learn more about the collaborations that are moving us closer to these goals.

Reagents for PD
Pathogenesis of PD may be related to environmental and genetic factors, and exposure to toxins and gene mutations may underlie formation of brain lesions. Several mechanisms of PD pathology have been elucidated, including α-synuclein aggregation, oxidative stress, ferroptosis, mitochondrial dysfunction, neuroinflammation, and gut dysbiosis, while other key implicated proteins are being identified.
α-Synuclein is an abundant protein in brain neurons where it occurs in multiple forms. The unfolded form is thought to help maintain supply of synaptic vesicles and release of dopamine, a neurotransmitter critical for regulating voluntary and involuntary movements. However, aggregation and propagation of misfolded α-Synuclein protein to other neurons is believed to underlie PD pathology.

Mechanism of chaperone-controlled regulation of α-Synuclein function, conformation, and localization in mammalian cells1.
Read below for our featured neuroscience reagents such as high-quality recombinant proteins for research into PD progression, immunoassays, and specific antibodies to detect native and aggregated forms of α-Synuclein.
MJFF Research Tools Catalog
To save researchers time and resources, The Michael J. Fox Foundation has made a number of tools available to the scientific community at low cost, with rapid delivery. The MJFF Research Tools Catalog is an interactive catalog that hosts all tools and models that MJFF makes available. Read below for our reagents featured on the catalog and designed for your research advances.
Learn more about the Research Tools Catalog >
Recombinant Proteins
Recombinant Human GAK (carrier-free)
Cyclin G-associated kinase (GAK), also known as auxilin 2, is a serine/threonine protein kinase that plays a crucial role in cellular processes, including membrane trafficking and cell cycle regulation. GAK regulates cyclin-dependent kinases and plays a key role in clathrin-mediated endocytosis via the interaction of the heat shock cognate protein 70. GAK is also involved in the regulation of protein degradation pathways, including the ubiquitin-proteasome system, and has emerged as a gene associated with Parkinson's disease.
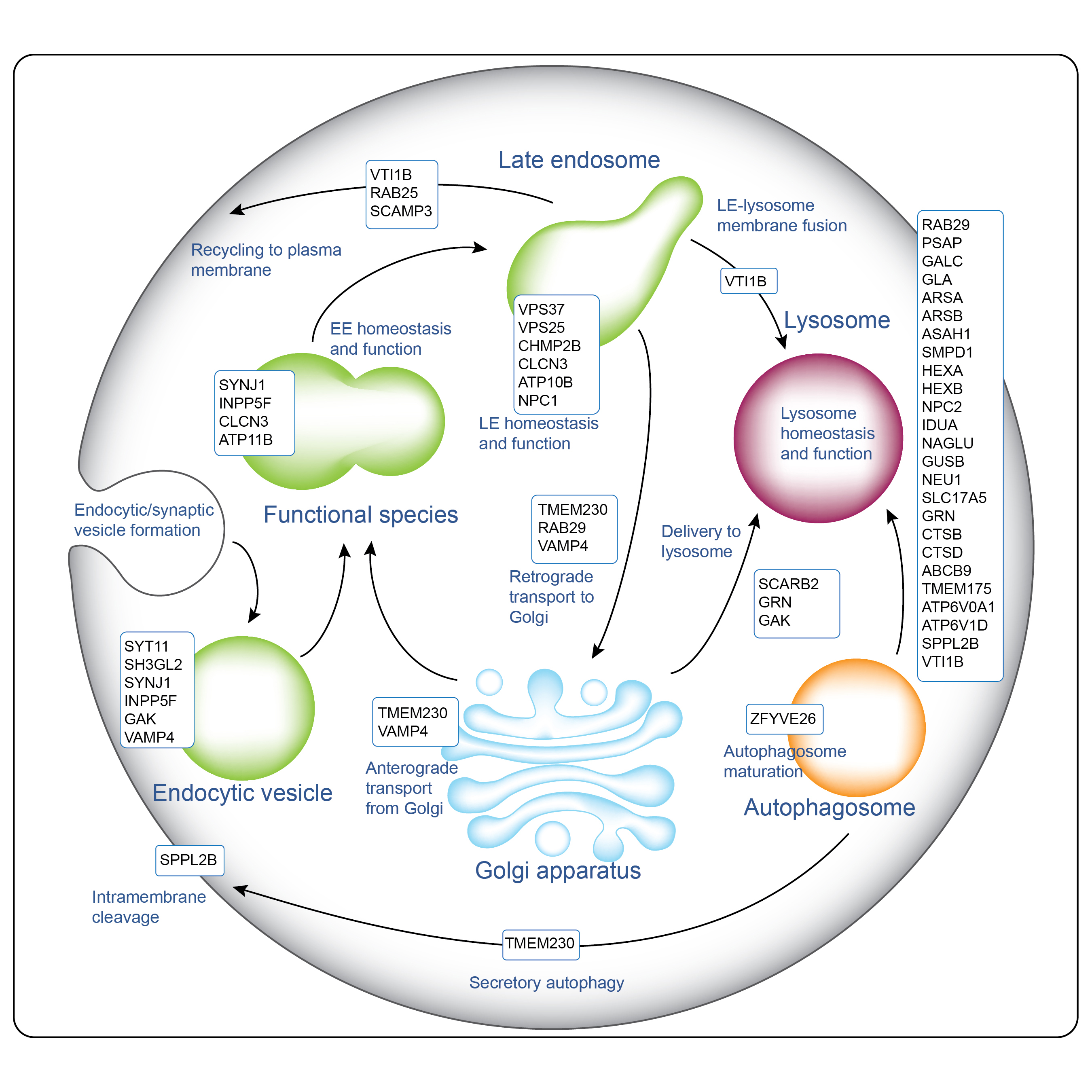
The endo-lysosomal system is emerging as a key player in the pathogenesis of PD2.
Recombinant Human INPP5F (carrier-free)
Inositol polyphosphate 5-phosphatase F (INPP5F), also known as phosphatidylinositide phosphatase SAC2, is a member of the INPP5 family. A key role of INPP5F is regulation of polyphosphoinositide metabolism and phosphoinositide signaling pathways by regulating the levels of inositol phospholipids. Positioned in the cytoplasm, INPP5F is closely associated with cellular membranes and participates in various cellular processes, including membrane trafficking, cytoskeleton dynamics, and signal transduction.
Mechanistically, INPP5F activates the Notch signaling pathway and upregulates c-MYC and cyclin E1 in hepatocellular carcinoma by interacting with ASPH. INPP5F also modulates the Akt/GSK-3β pathway by reducing Akt and GSK-3β phosphorylation levels. INPP5F is expressed in various tissues including heart, kidney, skeletal muscle, and brain. INPP5F contributes to tumorigenesis and tumor progression. INPP5F rs11789 6735 variant has been associated with an increased risk of Parkinson’s disease.
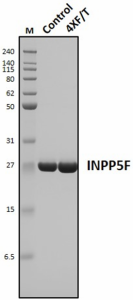
Stability testing for Recombinant Human INPP5F. One aliquot (Control) was stored at 4°C for 7 days, while another aliquot (4XF/T) was subjected to multiple freeze-thaw cycles by freezing at -80°C and thawing four times over a 7-day period. Each freeze-thaw cycle involved placing the sample on ice and slowly thawing it until no ice crystals remained in the tube. A minimum of 24 hours elapsed between each freeze and thaw event. Following treatment, the reduced samples (4 µg/lane) were separated by SDS-PAGE and stained with Coomassie brilliant blue dye. Lane M: Protein ladder (Cat. No. 773302).
Recombinant Human Cathepsin B (carrier-free)

Cathepsin B (CTSB) is a lysosomal cysteine protease. While most cathepsins are exclusively endopeptidases, CTSB exhibits both carboxypeptidase and endopeptidase activities. Cystatin C has been identified as an endogenous CTSB inhibitor. High CTSB protein levels and activities have been found in many tumors including breast, cervix, colon, stomach, glioma, lung, and thyroid tumors. CTSB can be secreted by tumor cells and is associated with the cell membrane of these cells. Membrane associated CTSB promotes extracellular matrix (ECM) degradation contributing to cancer motility and invasion. Many ECM proteins, including laminin, fibronectin, and collagen IV, are substrates of CTSB. CTSB can also activate pro-uPA/PLAU. Activated uPA promotes ECM digestion through serine protease plasminogen. Inhibition of CTSB can limit bone metastasis in breast cancer, making it an important anti-cancer drug target.
In animal models of PD, dysregulation of CTSB activity has been linked to neuronal cell death and the formation of Lewy bodies, a characteristic pathological feature of PD. CTSB has also been proposed as a new drug target for Alzheimer's disease because of its involvement in the production of β-amyloid (Aβ) peptides. Inhibition of CTSB was found to reduce brain Aβ peptides and improve memory in a mouse model with Alzheimer's disease.
CTSB also plays significant roles in immune responses including both T and B cell apoptosis, and Th1/Th2 polarization. CTSB is implicated in other pathological conditions including cardiovascular disease, multiple sclerosis, and arthritis.
Stability testing for Recombinant Human CTSB. One aliquot (Control) was stored at 4°C for 7 days, while another aliquot (4XF/T) was subjected to multiple freeze-thaw cycles by freezing at -80°C and thawing four times over a 7-day period. Each freeze-thaw cycle involved placing the sample on ice and slowly thawing it until no ice crystals remained in the tube. A minimum of 24 hours elapsed between each freeze and thaw event. Following treatment, the reduced samples (4 µg/lane) were separated by SDS-PAGE and stained with Coomassie brilliant blue dye. Lane M: Protein ladder (Cat. No. 773302).
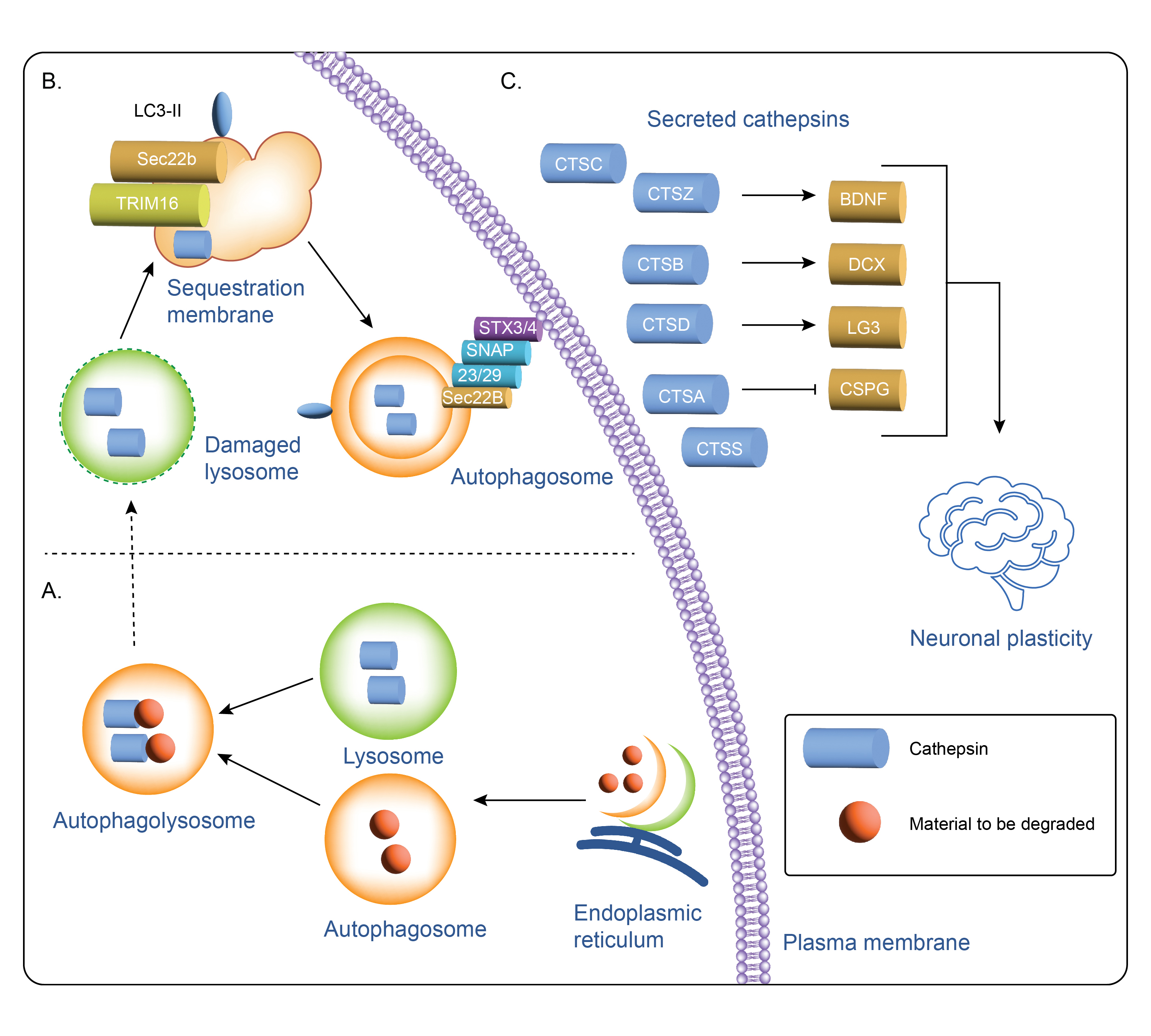
Cathepsin secretion and intracellular and extracellular cathepsin function3. Autophagosomes are generated at subdomains of the endoplasmic reticulum, (ER) where cathepsins carry out different intracellular functions (A). Cathepsin function in the secretory autophagy pathway (B) is mediated by several proteins including Sec22b and Syntaxin 3 (STX3) and 4 (STX4) leading to secretion and downstream extracellular cathepsin function (C).
Recombinant Human GPNMB (carrier-free)

GPNMB, also known as osteoactivin, is a type I transmembrane glycoprotein involved in various cellular functions, including cell migration, proliferation, invasion, adhesion, and differentiation. GPNMB regulates differentiation and activities of both osteoblasts and osteoclasts in bone development and plays a key role in the pathogenesis of autoimmune disease, neurodegenerative diseases, and various malignancies.
GPNMB is a risk gene for Parkinson’s disease through its interaction with α-synuclein. In induced pluripotent stem cell-derived neurons, loss of GPNMB resulted in loss of ability to internalize α-Syn fibrils and develop α-Syn pathology. GPNMB has also been shown to be protective in neurons undergoing active degeneration.
GPNMB is also expressed in certain cancers including melanoma, glioma, and breast cancer cells. In addition, GPNMB expression has been detected in dendritic cells, melanocytes, and a variety of tissues within skin, heart muscle, lung, lymph node, breast, and colon. Notably, GPNMB expressed in macrophages negatively regulates inflammation.
Stability testing for Recombinant Human GPNMB. One aliquot (Control) was stored at 4°C for 7 days, while another aliquot (4XF/T) was subjected to multiple freeze-thaw cycles by freezing at -80°C and thawing four times over a 7-day period. Each freeze-thaw cycle involved placing the sample on ice and slowly thawing it until no ice crystals remained in the tube. A minimum of 24 hours elapsed between each freeze and thaw event. Following treatment, the reduced samples (4 µg/lane) were separated by SDS-PAGE and stained with Coomassie brilliant blue dye. Lane M: Protein ladder (Cat. No. 773302).
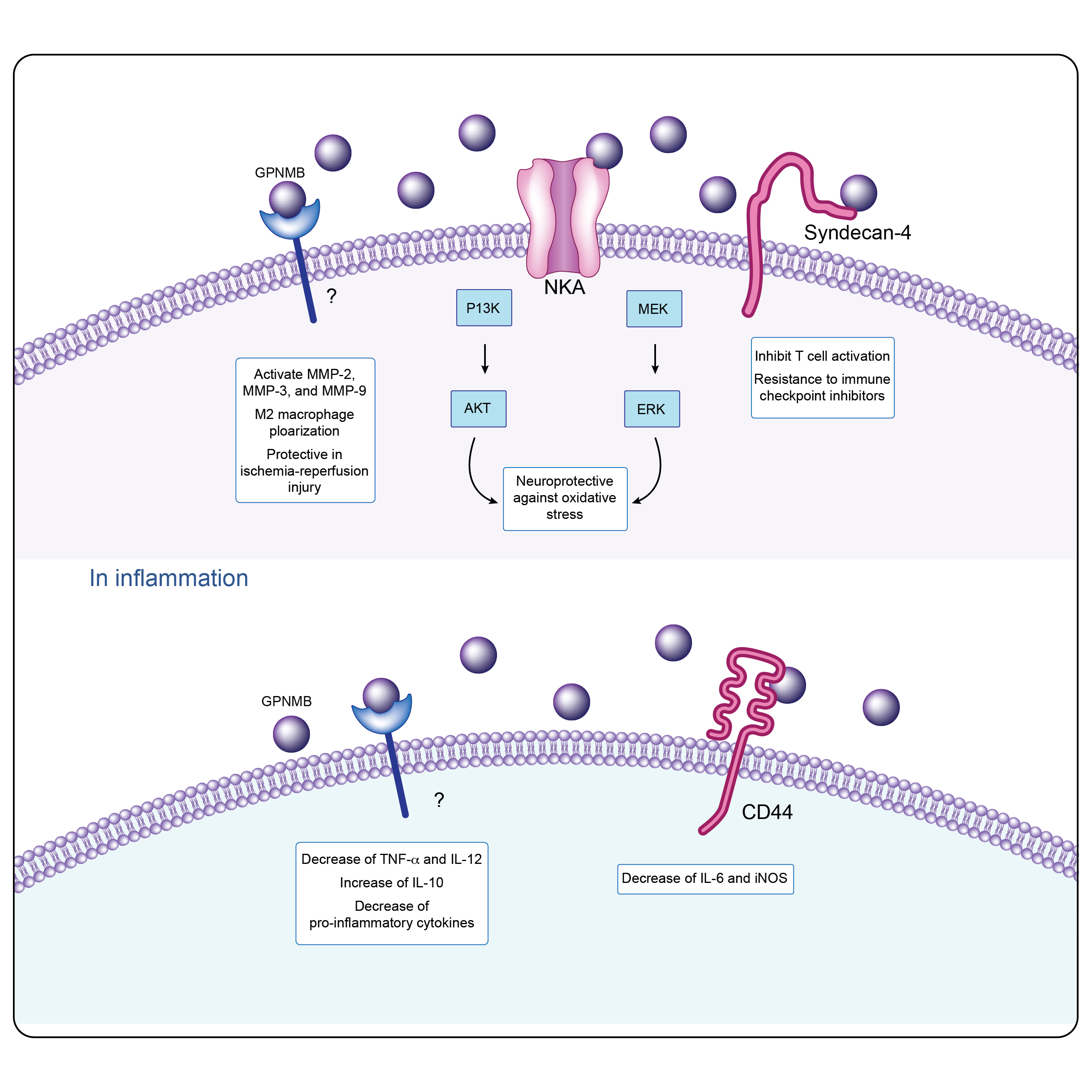
GPNMB intracellular signaling in physiological and inflammatory conditions4.
The release of a soluble GPNMB fragment, cleaved by the metalloproteinase ADAM10, releases a cascade of interactions with various molecular partners. These interactions include tyrosine kinase receptors, integrins, and heparan sulfate proteoglycans.
The role of GPNMB in inflammation is not clear due to it having both proinflammatory and anti-inflammatory roles. GPNMB's influence on adaptive immunity is mediated through its interaction with heparan sulfate proteoglycan syndecan-4. In the context of tumor biology, GPNMB binds to α5β1 fibronectin receptors, promoting tumor invasion and metastasis. GPNMB can be stimulated by various cytokines and growth factors, including granulocyte-macrophage-colony stimulating factors, Transforming growth factor- β, Interleukin-10, and interferon-γ.
Immunoassays
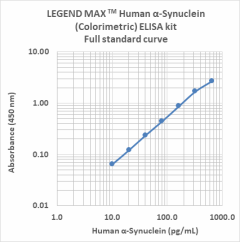
Our highly specific LEGEND MAX™ ELISA kits are analytically validated with ready-to-use reagents. The LEGEND MAX™ Human α-Synuclein (Colorimetric) ELISA Kit comes with a 96-well strip plate that is pre-coated with anti-human α-Synuclein monoclonal antibody. The detection antibody is a biotinylated anti-human α-Synuclein monoclonal antibody. This kit is specifically designed for the accurate quantitation of human α-Synuclein from CSF, serum, plasma, urine, tissue lysate, and cell culture supernatant.
Representative sandwich immunoassay standard curve generated using serial dilutions of LEGEND MAX Human α-Synuclein (Colorimetric) ELISA kit. Standard ranging from 10.2 pg/mL to 650 pg/mL.
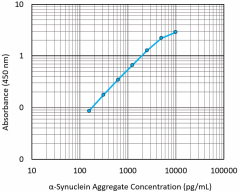
Our LEGEND MAX™ α-Synuclein Aggregate ELISA Kit is pre-coated with rat monoclonal anti-α-synuclein aggregate antibody. The detection antibody is a biotinylated goat polyclonal anti-α-synuclein aggregate antibody. This kit is specifically designed for the accurate quantitation of α-synuclein aggregates in tissue and cell lysates (e.g. human brain lysates, transgenic mouse brain lysates) and human cerebrospinal fluid.
Representative sandwich immunoassay standard curve generated using serial dilutions of LEGEND MAX α-Synuclein Aggregate standard ranging from 156.25 pg/mL to 10,000 pg/mL.

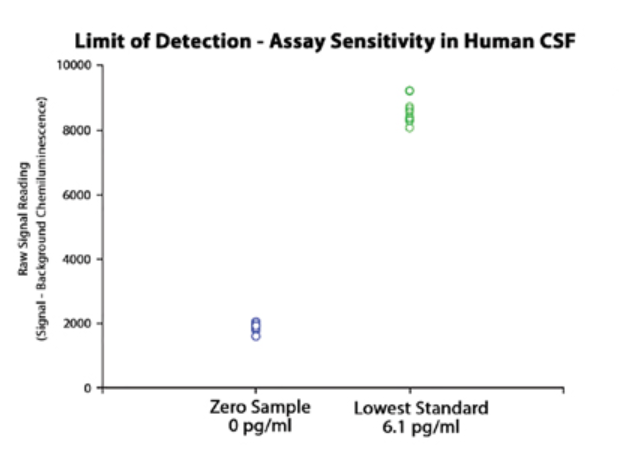
LEGEND MAX α-Synuclein Aggregate ELISA analysis showing assay recovery and sensitivity.
Featured antibodies
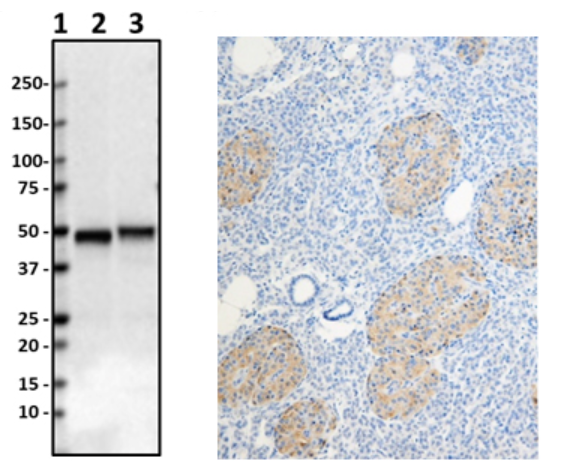
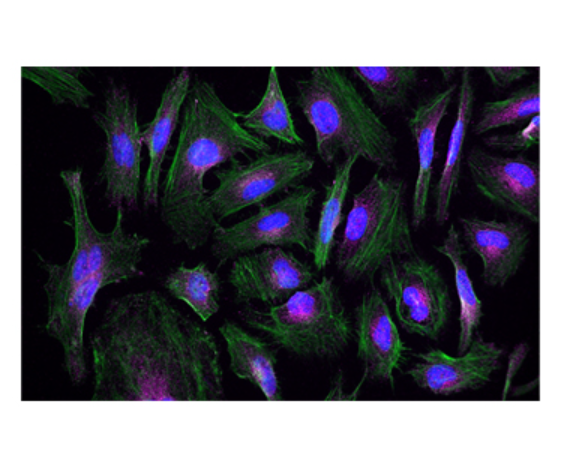
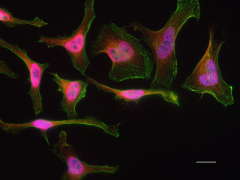
Anti-Parkin (clone 5C3/Parkin), tested by western blotting on human and rat brain lysates (left). IHC staining of purified anti-LRRK2 antibody (clone MC.028.83.76.242) on the formalin-fixed paraffin-embedded human pancreas tissue (middle). ICC staining of purified anti-Rab8 antibody (clone A18073A) on HeLa cells (right).
Parkin (also known as PARK2, AR-JP, PRKN) is a protein encoded by the PARK2 gene in humans. It is a component of the E3 ubiquitin ligase complex that mediates the targeting of proteins for degradation. Mutations in the PARK2 gene cause a familial form of Parkinson’s disease called autosomal recessive juvenile parkinsonism.
IHC staining of Purified anti-Parkin antibody (clone A15165D) on formalin-fixed paraffin-embedded PD substantia nigra tissue. Following antigen retrieval using Sodium Citrate H.I.E.R., the tissue was incubated with 10 µg/mL of the primary antibody overnight at 4°C. BioLegend’s Ultra Streptavidin (USA) HRP Detection Kit (Multi-Species, Cat. No. 929501) and Boost DAB Enhancer (Cat. No. 926701) were used for detection followed by hematoxylin counterstaining, according to the protocol provided. The image was captured with a 40X objective. Scale bar: 50 µm.
Rab8 is a small GTP/GDP binding protein that belongs to the RAS superfamily. It is well established that Rab8 is an important participant of macroautophagy, and is essential for several apical transport pathways. Recruitment of Rab8a and TBK1 by the autophagy receptor optineurin initiates the autophagy process at specific protein aggregate site, which leads to the clearance of aggregated protein.
ICC staining of Alexa Fluor® 594 anti-Rab8 antibody (clone A18073A) on HeLa cells. Cells were incubated with the primary antibody overnight at 4°C. Flash Phalloidin™ Green 488 (Cat. No. 424201) was used to stain the F-actin cytoskeleton. The slide was mounted with fluoromount-G with DAPI. The image was captured with a 60X objective. Scale bar: 20 µm.
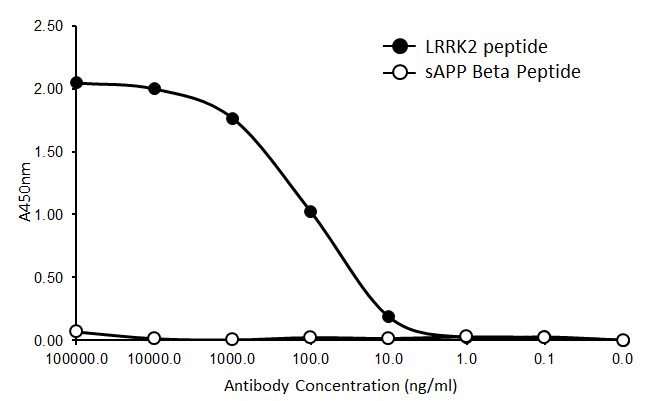
Leucine-rich repeat kinase 2 (LRRK2), is a member of the leucine-rich repeat kinase family and functions as a GTPase. Proteins with leucine-rich regions play a role in protein-protein interactions and mediate assembly of the cell's cytoskeleton. Mutations in LRRK2 genes have been associated with late-onset Parkinson's disease.
Direct ELISA of purified ant-LRRK2 antibody (clone MC.028.83.76.242). Wells were coated with 100 ng of plate-immobilized recombinant human LRRK2 peptide or sAPP Beta peptide. The wells were then incubated with the primary antibody at 37°C for 60 minutes, followed by incubation with the horseradish peroxidase labeled goat anti-mouse secondary antibody. TMB (Cat. No. 421501) was used as the detection system.
Clones for recognizing aggregated α-Synuclein
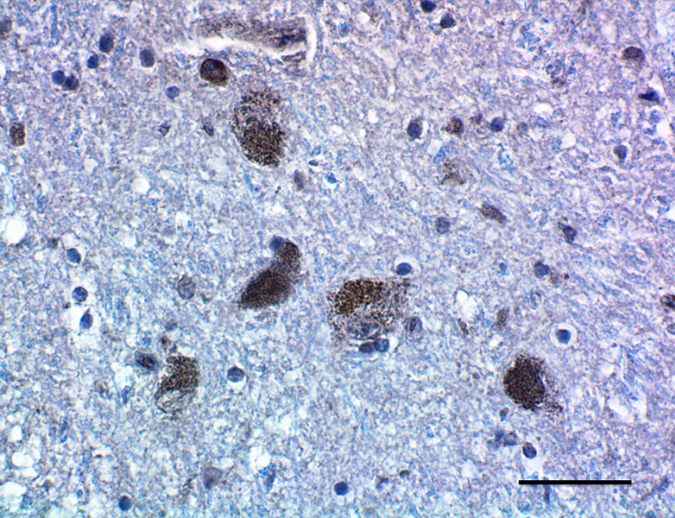
Having reagents that can differentiate between aggregated and non-aggregated forms of proteins is vital in researching neuropathies. To this end, we’ve created a number of in-house clones that recognize aggregated α-Synuclein (clones A17183A, A17183B, and A17183G). Each clone has been validated for FFPE PD brain tissue in IHC and demonstrates no cross-reactivity to native forms of α-Synuclein, β- and γ-Synucleins or β-Amyloid (Aβ 1-42).
As more markers and methods are discovered, we remain determined to create the reagents and resources needed to advance all aspects of your PD and neuroscience research, including useful protocols for whole mouse brain processing, astrocyte isolation and culturing, and our complimentary resource guide. Make a difference in your neuroscience research by trusting the high-quality and validated reagents created by our scientists.
Partner with us to find your research solution.
References
1. Burmann, Björn M., et al. "Regulation of α-synuclein by chaperones in mammalian cells." Nature 577.7788 (2020): 127-132.
2. Muraleedharan, Amitha, and Benoît Vanderperre. "The endo-lysosomal system in Parkinson’s disease: Expanding the horizon." Journal of Molecular Biology (2023): 168140.
3. Niemeyer, Christine, et al. "The role of cathepsins in memory functions and the pathophysiology of psychiatric disorders." Frontiers in Psychiatry 11 (2020): 718.
4. Saade, Marina, et al. "The role of GPNMB in inflammation." Frontiers in immunology 12 (2021): 674739.
 Login/Register
Login/Register 






Follow Us