MojoSort™ Human Pan DC Isolation Kit Column Protocol
Introduction
BioLegend MojoSort™ nanobeads work in commonly used separation columns, based on our internal research as well as validation by external testing by academic labs. This simple protocol consists of following the MojoSort™ protocol to label the cells with pre-diluted MojoSort™ reagents and using the columns as indicated by the manufacturer.
Note: Due to the properties of our beads, it may be possible to use far fewer beads that with other commercial suppliers. We recommend a titration to find the best dilution factor. However, as a general rule, dilutions ranging from 1:3 to 1:20 for the Nanobeads can be used. Please contact BioLegend Technical Service (tech@biolegend.com) if further assistance is needed.
Important Note
MojoSort™ magnetic particles can be used with other commercially available magnetic separators, both free standing magnets and column-based systems. Because MojoSort™ protocols are optimized for the MojoSort™ separator, the protocols may need to be adjusted for other systems. Please contact BioLegend Technical Service (tech@biolegend.com) for more information and guidance. We do not recommend using MojoSort™ particles for BD’s IMag™ or Life Technologies’ DynaMag™.
Protocol Steps
- Prepare cells from your tissue of interest or blood without lysing erythrocytes.
- In the final wash of your sample preparation, resuspend the cells in MojoSort™ Buffer by adding up to 4 mL in a 5 mL (12 x 75 mm) polypropylene tube.
Note: Keep MojoSort™ Buffer on ice throughout the procedure. - Filter the cells with a 70µm cell strainer, centrifuge at 300xg for 5 minutes, and resuspend in an appropriate volume of MojoSort™ Buffer. Count and adjust the cell concentration to 1 x 108 cells/mL.
- Aliquot 100µL of cell suspension (107 cells) into a new tube. Add 10µL Human TruStain FcX™. Mix well and incubate at room temperature for 10 minutes. Scale up the volume accordingly if separating more cells. For example, if the volume of Human TruStain FcX™ for 1x107 cells is 10µL, add 100µL for 1 x 108 cells. When working with less than 107 cells, use indicated volumes for 107 cells.
- To make the pre-diluted biotin, mix 10µL of the Biotin-Antibody Cocktail with 30µL of 1X MojoSort™ Buffer. Add 10µL of this pre-diluted Biotin-Antibody Cocktail. Mix well and incubate on ice for 15 minutes. Scale up the volume if separating more cells. For example, add 100µL of pre-diluted Antibody Cocktail for separating 1 x 108 cells in 1mL of MojoSort™ Buffer. When working with less than 107 cells, use indicated volumes for 107 cells.
- Vortex the Streptavidin Nanobeads (to resuspend) at max speed, 5 touches. Prepare the nanobead dilution by mixing 20µL of Streptavidin Nanobeads with 80µL of 1X MojoSort™ Buffer. Add 20µL of pre-diluted Streptavidin Nanobeads. Mix well and incubate on ice for 15 minutes. Scale up the volume accordingly if separating more cells. For example, add 200 µL of pre-diluted Nanobeads for separating 1 x 108 cells in 1 mL of MojoSort™ Buffer. When working with less than 107 cells, use indicated volumes for 107 cells.
- Wash the cells by adding MojoSort™ Buffer up to 4mL. Centrifuge the cells at 300xg for 5 minutes.
- Discard the supernatant.
- Resuspend the cells in appropriate amount of MojoSort™ Buffer and proceed to separation. At least 500µL is needed for column separation.
Note: There are several types of commercially available columns, depending on your application. Choose the one that fits best your experiment:
Columns:
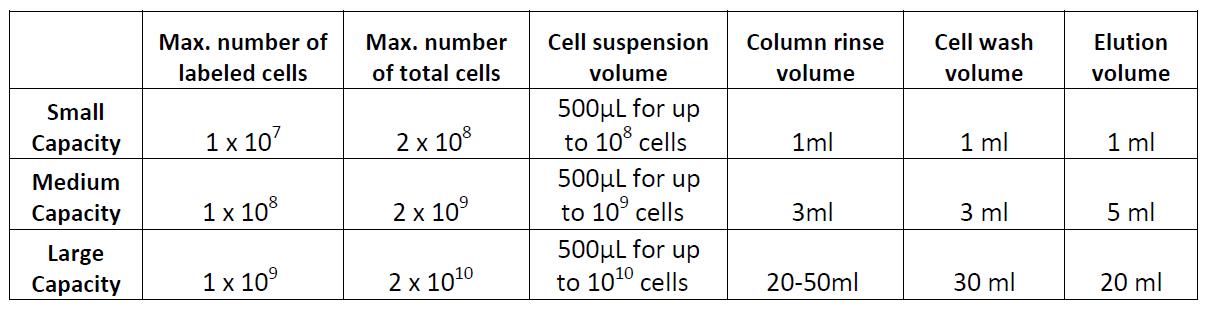
Example of magnetic separation with medium capacity columns:
- Place the column in a magnetic separator that fits the column.
- Rinse the column with 3 mL of cell separation buffer.
- Add the labeled cell suspension in at least 500uL of buffer to the column through a 30µm filter and collect the fraction containing the unlabeled cells. These are the cells of interest; do not discard.
- Wash the cells in the column 2 times with 3mL of buffer and collect the fraction containing the untouched cells. Combine with the collected fraction from step 3.
- If desired, the labeled cells can be collected by taking away the column from the magnet and place it on a tube. Then add 5 mL of buffer and flush out the magnetically labeled fraction with a plunger or supplied device. The labeled cells may be useful as staining controls, to monitor purity/yield, or other purposes.
Data
Flow cytometry. High purity and yield. “After Isolation” plot shows purified population of interest using pre-diluted MojoSort™ reagents in separation columns.

| Kit | Purity | Yield |
|---|---|---|
| Mouse CD4 T Cell Isolation Kit | 95.7% | 85% |
Electron Microscopy. CD4+ T cells Isolated with MojoSort™ CD4 T Cell Isolation Kit using columns do not display particles in the cell surface. Image is representative of 36 different cells.
 CD4+ T cells isolated with MojoSort™ CD4 T Cell Isolation Kit using separation columns. |
General Tips & FAQ
What dilution of the reagents shall I use?
Dilutions are lot dependent please contact tech@biolegend.com for recommended dilution.
 Login/Register
Login/Register 







Follow Us