- Other Names
- Xeno-free, Serum-free Human Serum Replacement
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

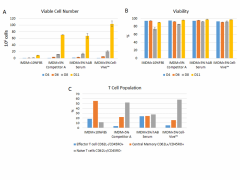
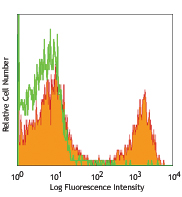
Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP can lead to higher PBMC derived T cell expansion, in particular of Naïve T Cells. PBMC-derived T cells were activated with 1 µg/mL of Ultra-LEAF™ anti-human CD3 antibody (Cat. No. 317347), 1 µg/mL of Ultra-LEAF™ anti-human CD28 antibody (Cat. No. 302943) in IMDM basal media supplemented with 200 IU/mL Recombinant human IL-2 (791908) and appropriate supplements. Cell number (A) and viability (B) were determined at day 4, 6, 8 and end of culture (day 11). Flow cytometry was used to determine specific T cell populations, by making use of CD62L and CD45RO cell markers (C). -

Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP can lead to higher PBMC derived NK cell expansion. PBMC-derived T cells were cultured in IMDM basal media supplemented with 1000 IU/mL Recombinant human IL-2 (791908) and appropriate supplements, in the presence of inactivated K562 feeder cells at ratio of (2:1). Cell number (A) and viability (B) were determined at day 14. C) Specific NK cell expansion was determined by combining total viable cell number generated with the % of NK cells. NK cell population was identified by flow cytometry as being CD3- and CD56+ cells.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 420502 | 50 mL | 165€ | ||||
Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP is specifically formulated to support PBMC-derived T and NK cell expansion, resulting in equal or higher specific cell numbers compared to the use of human AB serum. It is a xeno-free, serum-free formulation, prepared without bovine or other animal components, intended to be combined with the basal media of choice, such as IMDM. It is suitable for use in combination with several T cell activation methods and is able to support T cell populations (CD4, CD8) comparable to human AB serum. When used under the appropriate conditions, this product can result in increased naïve T cell numbers. This GMP product is suggested for use in research and further manufacturing. The benefits of this serum substitute include:
•Effective for generation of naïve CD3+ T cells from culture.
•Effective for maintaining CD4/CD8 balance.
•Maintains high cell viability, >90%, throughout cell culture.
•Effective for NK cell expansion.
•Minimizes safety risks associated with human AB serum.
•Improved lot-to-lot consistency over human AB serum.
•Saves time and costs required to qualify human AB serum
BioLegend Cell-Vive™ GMP cell culture products are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include
- Low endotoxin level (<1 EU/mL)
- Mycoplasma and bacterial/fungal growth testing
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- QA review of released products
- Quality audits per ISO 13485:2016
Product Details
- Formulation
- Xeno-free, serum-free formulation
- Endotoxin Level
- < 1 EU/mL
- Storage & Handling
- Store at -20°C to -5°C. Thaw overnight at 4°C. If necessary product can be kept at 4°C up to two weeks.
- Application
-
Cell Culture
- Recommended Usage
-
Recommended use at 5% v/v in basal media, combined with 200 IU/mL of human recombinant IL-2. Product should be titrated (2.5-10%) to determine optimal performance for your culture conditions.
- Application Notes
-
Note: This product contains human-derived components.
Complete cell Culture Media Preparation:
Thaw Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP overnight at 4°C. The product can be stored at 4°C in the dark up to 2 weeks post-thaw. Do not refreeze after initial thaw. Presence of cloudiness in the product after thaw is normal, and relates to the high enrichment of the product. Make sure to mix product prior to use. Once the product is diluted into basal media, no cloudiness should be observed.
Dilute Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP to 5% v/v in IMDM and 200 IU/mL of recombinant human IL-2 (BioLegend, Cat. No. 791906 or equivalent). Alternatively, the product can be used in combination with your basal media of choice and cytokines of interest. To determine optimal performance, a titration of the product under your desired cell culture setup may be required.
Cell Culture Platform:
Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP can be used with tissue culture plates, flasks and the G-REX® system. If using a bag format, testing is recommended with the specific bag being used.
Cell Activation methods:
Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP can be used in combination with anti-human CD3 and CD28 antibodies in solution, plate-coated or conjugated to magnetic beads. Optimal values should be determined if using a different culture setup.
For the protocols below, complete media refers to IMDM with 5% v/v Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP and 200 IU/mL recombinant human IL-2 (BioLegend, Cat. No. 791906 or equivalent) for T cell culture, and 1000 IU/mL for NK cell culture.
1. Coating plates with anti-human CD3 and CD28 antibodies
Add 1 µg/mL of anti-human CD3 (OKT3 clone, BioLegend, Cat. No. 317326 or equivalent) and 1 µg/mL of anti-CD28 antibodies (CD28.2 clone, BioLegend, Cat. No. 302934 or equivalent) to PBS. Add solution to the vessel, at recommended volume (table below). Incubate for 2 hrs at 37°C in a tissue culture incubator (or overnight at 4°C, preventing evaporation with Parafilm®). Aspirate solution and wash twice with 1 volume of PBS. Wells are ready to be used. Do not let wells dry.
TABLE 1: Recommended volumes of coating solution composed of anti-human CD3 and CD28 antibodies.Vessel
Recommended volume
12- well plate
0.75 mL
6- well plate
2 mL
T25 flask
5 mL
T75 flask
15 mL
2. Soluble antibodies
Add 10 µg/mL of anti-human CD3 (OKT3 clone, BioLegend, Cat. No. 317326 or equivalent) and 5 µg/mL of anti-CD28 antibodies (CD28.2 clone, BioLegend, Cat. No. 302934 or equivalent) to complete media.
PBMC-Derived T cell culture protocol:- Equilibrate appropriate amount of complete media at 37°C for 10-15 min.
- Plate PBMC (fresh or frozen) at 1 x 106 cells/mL and incubate at 37°C and 5% CO2.
- Allow cells to get activated for 3-4 days.
- At day 3-4, dissociate cell clumps by pipetting until a single cell solution is observed.
- Determine cell density.
- Dilute cell culture to 0.25 - 0.5 x 106 cells/mL and incubate at 37°C and 5% CO2.
- Repeat steps 4-6 every 2 days.
- Monitor cell culture to make sure confluency is not reached. Pending donor-to-donor variability exponential expansion may require more frequent feeding. Complete media has been shown to support up to 4 x 106 cells/mL without impact on cell viability and quality.
- Follow with further processing and/or cell analysis.
PBMC-Derived NK cell culture protocol using K562 feeder cells:
- Start by inactivating K562 cells with mitomycin-C (20 µg/mL in K562 growth media) for 45 min at 37°C and 5% CO2.
- Spin down inactivated K562 cells at 300xg for 5 min. Wash cell pellet twice with fresh media, to remove any leftover mitomycin-C.
- Equilibrate appropriate amount of complete media at 37°C for 10-15 min.
- Plate PBMC (fresh or frozen) at 0.5 x 106 cells/mL and incubate at 37°C and 5% CO2.
- Add feeder cells (inactivated K562 cells (recommended value of 1 K562 cell to 2 PBMC cells; optimal value can be determined for desired culture setup).
- Allow NK cells to get activated for 2-3 days.
- Determine cell density.
- Dilute cell culture to 0.25 - 0.5 x 106 cells/mL and incubate at 37°C and 5% CO2.
- Repeat steps 4-6 every 2 days.
- Re-feed NK cell culture with freshly inactivated K562 cells at day 7.
- Repeat steps 4-6 every 2 days up to day 14 (or appropriate culture end).
- Follow with further processing and/or cell analysis.
G-REX® is a registered trademark of Wilson Wolf Corporation.
Parafilm is a registered trademark of Bemis Company, Inc. - Additional Product Notes
-
BioLegend has submitted a Device Master File (DMF) to the US Food & Drug Administration (FDA) for “Cell-Vive™ T-NK Xeno, Serum Substitute, GMP” product. Neither the FDA nor other outside party may have access to or release the DMF without the holder's consent as all information contained in a DMF is considered confidential to the holder.
As a BioLegend customer using our “Cell-Vive™ T-NK Xeno, Serum Substitute, GMP” product in a manufacturing process, we can provide a “Letter of Authorization (LOA) to you in support of a submission or filing that you have made to the FDA. To initiate the reference authorization of our DMF, please submit a request to BioLegend with the required information so we can provide a DMF Letter of Authorization to the respective FDA Center. Once we have your request, we will advise the FDA that a BioLegend DMF is relevant to its review and that the agency has our consent to support your submission. To submit a request please contact our Customer Service Department.
- Disclaimer
-
BioLegend Cell-Vive™ GMP Cell Culture products are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products.
Antigen Details
- Gene ID
- NA
Related Pages & Pathways
Pages
Customers Also Purchased
 Login / Register
Login / Register 













Follow Us