- Other Names
- Fc Receptor Blocking Solution
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

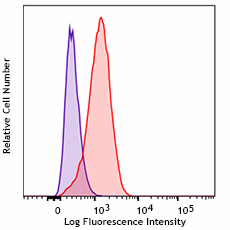
THP-1 cells were treated with Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP followed by staining with an irrelevant PE mouse IgG2a, κ Isotype (clone MOPC-173, purple histogram). Non-specific binding of the same antibody on untreated cells is shown in red.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 420510 | 100 tests | 220€ | ||||
Human Fc receptors (FcRs) are molecules that regulate the immune responses. FcRs are expressed by several hematopoietic cells. Due to FcRs-mediated Ig Fc binding, false positive or false negative results of immunofluorescent staining could occur. Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP is specially formulated to block the FcR-involved unwanted staining without interfering with the antibody-mediated specific staining of human cells. Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP is compatible with flow cytometric staining with anti-human CD16 (clone 3G8), CD32 (clone FUN-2), and CD64 (clone 10.1) antibodies. Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP contains ultra-low endotoxin levels (<0.01EU/µg of protein) and is tested negative for mycoplasma and microbial growths.
This GMP product is for research and further ex vivo bioprocessing use only.
BioLegend Cell-Vive™ GMP cell culture products are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include
- Low endotoxin level (< 1 EU/mL)
- Mycoplasma and bacterial/fungal growth testing
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- QA review of released products
- Quality audits per ISO 13485:2016
Product Details
- Formulation
- Serum-Free, Defined, containing no preservatives. Endotoxin level is < 0.01 EU/µg of the protein (< 0.001 ng/µg of the protein) as determined by the LAL test.
- Endotoxin Level
- < 1 EU/mL
- Storage & Handling
- Store between 2°- 8°C. Handle under aseptic conditions.
- Application
-
FC - Quality tested
- Recommended Usage
-
5 μl of Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP per million cells in 100 μl staining buffer.
- Application Notes
-
This buffer contains specialized human IgG. It is not recommended to be used for staining human IgG. Handle as biohazard agent. Add 5 μl of Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP per million cells in 100 μl staining buffer, mix and incubate at room temperature for 5-10 minutes prior to staining with antibody of interest. It is not necessary to wash cells between these blocking and immunostaining steps.
- Disclaimer
-
BioLegend Cell-Vive™ GMP Cell Culture products are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products.
Related Pages & Pathways
Pages
Related FAQs
Customers Also Purchased
 Login / Register
Login / Register 














Follow Us