- Regulatory Status
- RUO
- Other Names
- T4/Leu-3, B4, CVID3, T3, CD3ε, FcγRIII, Integrin αX subunit, CR4, p150, ITGAX, Leu-19, NKH1, Monocyte differentiation antigen CD14, myeloid cell-specific leucine-rich glycoprotein, LPS receptor, T8, Leu2, Leukocyte Common Antigen (LCA), T200
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

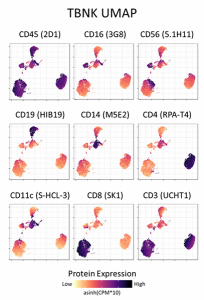
Major immune cell lineages are identified using the TotalSeq™-A TBNK Cocktail. Human peripheral blood mononuclear cells were stained with the TotalSeq™-A TBNK Cocktail and processed using the 10x Genomics Single Cell 3' v3 feature barcoding kit and Illumina sequencing. Protein count data were transformed and visualized in a UMAP projection overlaid with protein expression levels for each component of the cocktail. Cell clusters were identified based on protein expression only.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 399901 | 5 tests | £814 | ||||
The TotalSeq™-A Human TBNK Cocktail has been designed to react with immune cells as defined and classified by the expression of the surface antigens CD19, CD3, CD16, CD4, CD11c, CD56 (NCAM), CD14, CD8, CD45.
The lyophilized cocktail provides convenience when processing several samples at the same time or at different time points, reducing inconsistencies associated with pipetting and handling multiple vials and samples. It also provides optimized and titrated antibodies that are ready to use and packaged in convenient single-use vials.
Product Details
- Verified Reactivity
- Human
- Formulation
- Lyophilized from PBS containing stabilizers and sodium azide. Please reconstitute each tube with 50 µL of cell staining buffer when ready to use. Make sure the cake is completely dissolved prior to using the cocktail for staining cells. The lyophilized cocktail material can vary in appearance and appear as either a lyophilized cake or a powder.
- Preparation
- This reagent is a combination of TotalSeq™-A oligo conjugated clones at optimal concentrations for single-cell sequencing analysis. See additional product notes for a list of clones.
- Concentration
- Lot-specific (to obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.)
- Storage & Handling
- The lyophilized antibody cocktail should be stored between 2°C and 8°C in powder form and in a sealed container with desiccant until ready to use. Once vial is opened, it is to be reconstituted immediately. After reconstitution cocktail should be used within 2 hours.
- Application
-
PG - Quality tested
- Recommended Usage
-
This panel has been optimized on human PBMCs. Using lysed whole blood additional TotalSeq™ antibodies may require further optimization. Please contact our technical service group for further information.
Buyer is solely responsible for determining whether Buyer has all intellectual property rights that are necessary for Buyer's intended uses of the BioLegend TotalSeq™ products. For example, for any technology platform Buyer uses with TotalSeq™, it is Buyer's sole responsibility to determine whether it has all necessary third party intellectual property rights to use that platform and TotalSeq™ with that platform. - Application Notes
-
Protocol for using TotalSeq™-A Human TBNK Panel
Lyophilized Panel Reconstitution and Staining- Equilibrate the lyophilized panel vial(s) to room temperature for 5 minutes.
- Place lyophilized panel vial in an empty Eppendorf tube, spin down at 10,000 x g for 30 seconds at room temperature.
- Rehydrate lyophilized panel by adding 50ul of Cell Staining Buffer (BioLegend Cat. No. 420201). Replace the cap and vortex for 10 seconds.
- Incubate at room temperature for 5 minutes.
- Vortex again and spin down at 10,000 x g for 30 seconds at room temperature.
- Transfer the entire volume (50 µL) of reconstituted cocktail to a low protein binding Eppendorf tube (Fisher Cat. No. 022431081 or similar tube).
- Centrifuge at 14,000 x g for 10 min at 4°C.
- While cocktail is being centrifuged, block cells by adding 5 µL of Human TruStain FcX™ Fc blocking reagent (Cat. No. 422301, 422302) to 1x106 cells in 45 µL Cell Staining Buffer (total volume = 50 µL). Incubate for 10 min at 4°C. Perform staining in 12 x 75 mm tubes.
- Transfer 50 µL of reconstituted cocktail to the tube containing 50 µL of FcR blocked cells, taking care to avoid the bottom of the tube. The final staining volume is 100 µL.
- Proceed with corresponding staining protocol:
- For TotalSeq™-A panels, start at step 8 of the following protocol: go.biolegend.com/totalseq-a-v3-protocol
- For TotalSeq™-B or TotalSeq™-C panels, start at step 8 of the following protocol: go.biolegend.com/totalseq-b-c-protocol
- Additional Product Notes
-
TotalSeq™ reagents are designed to profile protein expression at a single cell level following an optimized protocol similar to the CITE-seq workflow. A compatible single cell device (e.g. 10x Genomics Chromium System and Reagents) and sequencer (e.g. Illumina analyzers) are required. Please contact technical support for more information, or visit biolegend.com/totalseq.
The flanking sequence is CCTTGGCACCCGAGAATTCCA, and the capture sequence is BAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA*A*A. B represents either C, G, or T, and * indicates a phosphorothioated bond, to prevent nuclease degradation.
The full oligomer sequence for this product, with the specific barcode in brackets is
CCTTGGCACCCGAGAATTCCA[Barcode]BAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA*A*A.
Download the excel file for a full list of clones and barcodes.
Download the Cell Ranger antibody reference and UMI counting csv file.
- RRID
-
AB_2820073 (BioLegend Cat. No. 399901)
Antigen Details
- Distribution
-
T cells, B cells, NK cells, NKT cells, monocytes
- Cell Type
- Antigen-presenting cells, B cells, Dendritic cells, Lymphocytes, Macrophages, NK cells, T cells
- Biology Area
- Immunology
- Molecular Family
- CD Molecules
- Gene ID
- NA
Related Pages & Pathways
Pages
Related FAQs
- What are TotalSeq™ reagents and how do they work with established workflows (CITE-seq, and REAP-seq)?
-
TotalSeq™ is BioLegend’s brand of antibody-oligonucleotide conjugates, to enable simultaneous analysis of proteins and mRNA in single cells. CITE-seq and REAP-seq are two similar workflows for simultaneous protein and mRNA analysis, and the TotalSeq™ conjugates integrate seamlessly into these established protocols.
- What is the difference between TotalSeq™-A, -B, and –C?
-
The main difference between the 3 formats relates to the sequences of the oligo tags and their downstream compatibility with single-cell sequencing platforms.
- TotalSeq™-A reagents use a poly-A capture method that is instrument agnostic which includes the 10x Genomics 3’ Single Cell Gene expression kit.
- TotalSeq™-B utilizes the feature barcode technology for 10x Genomics 3’ Single Cell Gene expression kit.
- TotalSeq™-C also uses the feature barcode technology but for 10x Genomics 5’ V(D)J expression kit.
They each use different primers for library amplification. You can read a little more about the differences on our TotalSeq™ webpage. - Can I use TotalSeq™-A and -B together?
-
We are not currently able to support the mixing of TotalSeq™-A and TotalSeq™-B reagents in a single experiment. These two product lines require different protocols for library prep, and the different capturing methods may cause discrepancies in capture efficiency. In addition, it can often cause issues downstream in the bioinformatics portion when analyzing your data due to the variations in index sequences and lengths. In theory, they should work together, but in practice it can be quite difficult and we advise against doing so whenever possible.
- Can I use TotalSeq™-A on the 10x Genomics 3’ Single Cell Gene platform or do I need TotalSeq™-B?
-
Either will work fine.
- What are “features”?
-
When discussing single-cell data, features are any aspect of the cell that is being measured. Common features are mRNA, protein, sgRNA, etc.
- Is TotalSeq™-A, B, and C supported by 10x Genomics?
-
TotalSeq™-A/B/C products are fully supported by BioLegend and have been internally tested by BioLegend on the 10x Genomics platform. TotalSeq™-B and C are fully supported by 10x Genomics. 10x Genomics provides limited support for TotalSeq™-A.
If you are unsure about which technical service team to contact if you have questions or issues, feel free to reach out to BioLegend and we will work with our partners to resolve your problem or answer your technical questions. - What are "Hashtags"?
-
Hashtag reagents are intended to be used for sample barcoding, which allows users to combine multiple samples into a single lane, then de-multiplex during analysis. Instead of select antigen-specific antibodies, the hashtags are designed so that they are specific for human or mouse cells, and to cover as many cells as possible. For human samples, the hashtags are made of two antibodies that recognize ubiquitous surface markers, CD298 and β2 microglobulin, each conjugated to the same oligonucleotide containing the barcode sequence. For mouse samples, the surface markers are CD45 and H-2 MHC class I. The conjugates are already pre-mixed and ready to use.
- What’s the benefit of Cell hashing?
-
Hashing allows customers to run multiple samples in a single lane of a 10x Genomics Chromium instrument, or equivalent, which optimizes the number of cells or samples that can be analyzed simultaneously. This can reduce variability due to batch effects or sample handling. It can also help to identify doublets more efficiently. Furthermore, it can also improve yield and help with cell input when cell numbers are low, therefore optimizing the use of the platform and your workflow or protocol.
- What is “super loading”?
-
Typically, when using a single-cell platform, as you increase the amount of input cells, the rate of doublets (two cells captured as one data point) increases. This leads to an unacceptable percentage of datapoints that contain more than one cell. Hashtags allows you to “super load” the number of cells. To this end, multiple aliquots of the same sample could be stained with as many hashtags as aliquots you’d wish to mix. The number of aliquots and number of cells per aliquot may vary, but after washing and mixing the cells, following the staining and analysis protocol, if more than one hashtag is detected in a single-cell “event”, that event can be safely discarded as a doublet. This does not eliminate doublets that contain the same hashtag but the rate of doublet detection is greatly increased. However, “super loading” should be carefully controlled as the more cells you load the more doublets occur, causing diminishing returns overall. There should be a balance between multi-sample optimization, single-cell data yield, and optimal instrument/platform performance.
- What are nuclear hashtags?
-
Nuclear hashtags are used for single-nucleus RNA-seq (snRNA-seq) samples. snRNA-seq captures RNAs that are isolated with the nucleus. This is done because whole, intact cells may be difficult to isolate due to specific experimental or sample conditions, or to answer a specific scientific question. See an example of nuclear hashtag application in this paper: https://www.nature.com/articles/s41467-019-10756-2
- Can I use nuclear hashtags for single cell ATAC-seq?
-
Unfortunately, our TotalSeq™ reagents are not directly compatible with single ATAC-seq kits at this time. However, the NYGC has pioneered a bridging method ATAC with Select Antigen Profiling by sequencing (ASAP-seq) which is able to bridge TotalSeq™ antibodies with other capture platforms such as scATAC-seq. https://www.biorxiv.org/content/10.1101/2020.09.08.286914v1
Our nuclear hashtag products available off the shelf, but we do not currently have a recommended protocol for this application at this time.
The only recommendations we can provide are literature references such as this one:
https://www.nature.com/articles/s41467-019-10756-2 - Can I get technical support when using hashtags?
-
Hashtags can absolutely be used in any single cell platform including the 10x Genomics platform. BioLegend fully supports the usage of hashtags. Please contact our technical service group for more information.
- Does co-staining for FACS and TotalSeq™ interfere with the workflow?
-
We have not tested this, but there is no reason to believe that non-competing clones for the same target would be problematic. Similarly, a sub-saturating concentration of both reagents against the same target should be tolerated by the cell/technique. The original CITE-seq paper by Stoeckius et al.(Fig. 2) demonstrated that cells can be co-stained for downstream analyses. Although the authors did not use current TotalSeq™ reagents, the technology is the same. In addition, other CITE-seq users have also demonstrated this approach. Please contact our technical service group for more information.
- Why is it essential to remove dead cells prior to subjecting samples for CITE-seq?
-
We recommend >95% viability before beginning you CITE-seq experiment. Running dead cells during CITE-seq essentially leads to “wasting” single cell runs on dead cells, which may ultimately lead to sub-optimal data, or not processing enough cells to pick up the positive events, especially if the frequency of your target cell is very low. Dead cells can also be removed using magnetic beads. If performing CITE-seq before eliminating dead cells is required, it is possible to use bioinformatics methods to try to clean up low quality events (which may include dead cells). This could be a more complex approach, but in such cases, we recommend the following reading:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4758103/
https://www.ncbi.nlm.nih.gov/pubmed/28045081 - Is it necessary to sort samples prior to CITE-seq? What should I consider when deciding to pre-sort, or not?
-
If the populations of interest can be clustered/identified without sorting, the likelihood that you need to pre-sort is minimal. However, if the cell number in the cluster is very small, it may not be sufficient to draw conclusions when compared to control, or other cell populations. In which case, enrichment of this population prior to CITE-seq analysis may help with obtaining more meaningful sequencing data that is selective for your rare cell population(s) of interest. We also recommend to sort out dead cells if the viability in a sample is lower than 95%.
- How can I titrate TotalSeq™ antibodies?
-
We have developed pre-titrated, lyophilized TotalSeq™ panels to facilitate the use of combined antibodies. We have panels in all three formats (TotalSeq™-A, B, and C) designed to identify the main immune cells, as well as a universal panel that contains antibodies against 130 protein targets, and 7 isotype controls. We will keep on releasing these pre-optimized panels as we understand that titrating dozens of antibodies for this application is not an easy task. We may not be able to provide specific titration values for individual antibodies as that may depend on multiple factors. However, here are some recommendations in case you may not be able to use our panels, and need to perform your own titrations:
- Performing titrations using the actual CITE-seq method is costly, but using hashtags can make the process more affordable. See Fig. 3 and associated methods of this publication for more information.
- Perform flow cytometry experiments to find the optimal antibody concentration, using the same clone conjugated to PE. The flow cytometry antibody amount should translate well to CITE-seq.
- Label the cells with different concentrations of a TotalSeq™ antibody, and then use a poly(dT) oligo conjugated to a fluorophore, such as Alexa Fluor® 647, as a secondary reagent to detect the TotalSeq™ antibody. This is a more direct method, but it requires the use of the actual TotalSeq™ antibody.
- What tools are available for data analysis?
-
Some available resources include CITE-Seq-Count, and the tools developed by the Rahul Satija laboratory. Please contact our technical service group for further information.
- I need to order primers; where do I order them and what purity is needed?
-
There are several companies offering primer synthesis. We can recommend IDT technologies. For additive primers, desalt purification is sufficient. For index primers, either HPLC or PAGE is needed.
- Why should I generate ADT/HTO libraries separate from mRNA?
-
Separate ADT/HTO and mRNA libraries provide flexibility when sequencing. Libraries can be mixed at different ratios before sequencing depending on the number of reads needed. Typically, ADT/HTO libraries require less sequencing depth compared to their mRNA counterparts.
- How is the oligo tag bound to the antibody so that it does not interfere with antibody binding to target protein?
-
The oligo is attached to the antibody in the same method as some of our fluorophore-conjugated antibodies that are quality tested by flow cytometry. Once the antibodies have been conjugated to the oligonucleotide, we verify by flow cytometry that the antibody can still bind to its target. This is part of our quality control process for TotalSeq™ conjugates.
- Is it possible to run CyTOF® and TotalSeq™ (CITE-seq) alongside each other?
-
Not in the same experiment; it is not possible because CyTOF® requires its own instrument and protocol, and it destroys the sample in the process. To do so, it is necessary to split the sample and run both CyTOF® and CITE-seq assays. Note that CITE-seq generates equivalent data for surface proteins as CyTOF® with higher panel multiplexing capabilities and at a single-cell level.
- Can CITE-seq be extended to micro-RNAs?
-
This is a technically challenging application as micro-RNAs are very small, and in many cases will be as small as or even smaller than the required primers to execute the protocol. It is not possible at the moment.
- Can I use TotalSeq™ in bulk RNA-seq experiments?
-
Yes, the protocol to perform bulk epitope and nucleic acid sequencing (BEN-seq) has been developed as a collaboration between Illumina and BioLegend. You can read more about it in our application note.
- Is autofluorescence an issue as in flow cytometry?
-
No, sequencing as the final readout is not affected by cell autofluorescence.
- What is the baseline copy number for detection of protein expression (via TotalSeq™ labeling)?
-
This has to do mostly with antibody affinity, titration, and level of antigen expression. BioLegend and some collaborators are working on titration data. There is also some data already published. In theory, as long as the antibody can bind one molecule, PCR amplification and sequencing should pick it up, but there is always background noise. A common way to control for this is to add negative cells to your experiment. For example, if working with human PBMCs, use mouse cells and vice versa.
- How many cells do you need per experiment?
-
This depends on the biological question that needs to be answered. From the technical perspective, when using 10x Genomics Chromium, it is recommended to load 5,000 – 10,000 cells per lane. The use of hashtags can increase that number. For ease of staining, we typically recommend 1 million cells, but this number also depends on availability of cells and the operator’s ability to handle low cell numbers.
- How many TotalSeq™ antibodies is it possible to multiplex?
-
We have optimized multiple pre-mixed antibody cocktails, including TotalSeq™-A, -B, or -C Human TBNK that contain 9 antibodies and our TotalSeq™-C Human Universal Cocktail, v1.0 which contains an unprecedented 130 target antibodies plus 7 isotype controls in a single vial. We will be releasing more panels in the near future. The literature suggests that panels larger than these cocktails are possible. For example, Yapeng et al. published a study using 197 TotalSeq™ antibodies and 7 isotype controls. BioLegend and the scientific community keep pushing the upper limits of multiplexed protein detection by sequencing and have yet to find one.
- Is it possible to do FACS sorting first then do CITE-Seq, while performing both antibody-oligo and antibody-fluorophore labelling at the same time? Will the oligo conjugate withstand sorting?
-
The oligo should withstand sorting. However, based on theoretical considerations we recommend using non-competing clones, to minimize impact in either FACS or sequencing signal/reading. Even if exposed for a very short time, we have not evaluated the effect of ultraviolet or violet lasers on the oligo conjugates. An alternative to flow sorting could be negative magnetic bead enrichment, such as our MojoSort™ platform.
- What should be my sequencing depth be for my ADT/HTO library?
-
We recommend a sequencing depth of 5,000 reads/cell. If you are using more than 100 TotalSeq™ antibodies, you should increase to 10,000 reads/cell. For HTOs alone, 500 reads/cell is sufficient.
- Do I need isotype controls?
-
Isotype controls are highly recommended but not required. The utility of isotype controls in these applications is that they can help identify “sticky cells” caused by cells with compromised viability. Isotype controls also provide an overall picture of the background level or non-specific binding of different cell types. Typically when running isotype controls for CITE-seq assays, you want to include 1 isotype control for every isotype included in the panel, regardless of how many antibodies there are. We recommend using the isotype control at a concentration matching the highest concentration of a single antibody used in a panel, for that particular isotype. Typically, the isotype controls should be used in the same tube as the panel itself. Unlike a traditional flow cyometry experiment, the isotype controls for multiomics cell characterization do not need their own separate samples for comparison. Isotype controls are included in our TotalSeq™ Universal Cocktails.
Another useful control to help identify positive signals are cells known to not bind the antibodies used. For example, murine cells when characterizing human samples, as long as the antibodies do not cross-react between mouse and human targets. This, however, is not required to confidently identify positive signals. - Should I use dextran sulfate to block?
-
Several genomics protocols, including some from 10x Genomics, may recommend including dextran sulfate as a blocker to prevent unwanted charge interactions between nucleic acids and other positively charged molecules. However, we have found that in some cases, the inclusion of dextran sulfate can interfere with antibody binding and recognition and we do not recommend using it with any of our TotalSeq™ reagents. It’s unclear what is the exact mechanism of action that causes this issue. All our protocols reflect this observation; thus we do not recommend the use of dextran sulfate.
 Login / Register
Login / Register 














Follow Us