- Clone
- PK136 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- NKR-P1C, NKR-P1B, Ly-55, CD161, CD161b, CD161c
- Isotype
- Mouse IgG2a, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

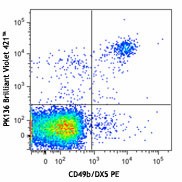
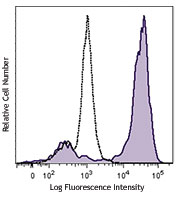
C57BL/6 mouse splenocytes were stained with CD49b/DX5 PE and NK1.1 (clone PK136) Brilliant Violet 421™ (top) or mouse IgG2a, κ Brilliant Violet 421™ isotype control (bottom). -

-
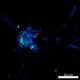
Confocal image of C57BL/6 mouse liver sample acquired using the IBEX method of highly multiplexed antibody-based imaging: CD8 (cyan) in Cycle 2, CD44 (blue) in Cycle 2, and NK1.1 (red) in Cycle 3. Tissues were prepared using ~1% (vol/vol) formaldehyde and a detergent. Following fixation, samples are immersed in 30% (wt/vol) sucrose for cryoprotection. Images are courtesy of Drs. Andrea J. Radtke and Ronald N. Germain of the Center for Advanced Tissue Imaging (CAT-I) in the National Institute of Allergy and Infectious Diseases (NIAID, NIH). -
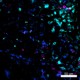
Mice were injected subcutaneously with sheep red blood cells in a volume of 25 µl per site on days 0 and 4 and harvested on day 11. Confocal image of C57BL/6 mouse lymph node acquired using the IBEX method of highly multiplexed antibody-based imaging: F4/80 (cyan) in Cycle 3, CD68 (blue) in Cycle 6, and NK1.1 (magenta) in Cycle 9. Tissues were prepared using ~1% (vol/vol) formaldehyde and a detergent. Following fixation, samples are immersed in 30% (wt/vol) sucrose for cryoprotection. Images are courtesy of Drs. Andrea J. Radtke and Ronald N. Germain of the Center for Advanced Tissue Imaging (CAT-I) in the National Institute of Allergy and Infectious Diseases (NIAID, NIH).
NK-1.1 surface antigen, also known as CD161b/CD161c and Ly-55, is encoded by the NKR-P1B/NKR-P1C gene. It is expressed on NK cells and NK-T cells in some mouse strains, including C57BL/6, FVB/N, and NZB, but not AKR, BALB/c, CBA/J, C3H, DBA/1, DBA/2, NOD, SJL, and 129. Expression of NKR-P1C antigen has been correlated with lysis of tumor cells in vitro and rejection of bone marrow allografts in vivo. NK-1.1 has also been shown to play a role in NK cell activation, IFN-γ production, and cytotoxic granule release. NK-1.1 and DX5 are commonly used as mouse NK cell markers.
Product DetailsProduct Details
- Reactivity
- Mouse
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Immunogen
- NK-1+ cells from mouse spleen and bone marrow
- Formulation
- Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide and BSA (origin USA).
- Preparation
- The antibody was purified by affinity chromatography and conjugated with Brilliant Violet 421™ under optimal conditions.
- Concentration
- µg sizes: 0.2 mg/mLµL sizes: lot-specific (to obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.)
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C, and protected from prolonged exposure to light. Do not freeze.
- Application
-
FC - Quality tested
SB - Reported in the literature, not verified in house
- Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For immunofluorescent staining using the µg size, the suggested use of this reagent is ≤ 0.5 µg per million cells in 100 µl volume. For immunofluorescent staining using µl sizes, the suggested use of this reagent is 5 µl per million cells in 100 µl staining volume or 5 µl per 100 µl of whole blood. It is recommended that the reagent be titrated for optimal performance for each application.
Brilliant Violet 421™ excites at 405 nm and emits at 421 nm. The standard bandpass filter 450/50 nm is recommended for detection. Brilliant Violet 421™ is a trademark of Sirigen Group Ltd.
Learn more about Brilliant Violet™.
This product is subject to proprietary rights of Sirigen Inc. and is made and sold under license from Sirigen Inc. The purchase of this product conveys to the buyer a non-transferable right to use the purchased product for research purposes only. This product may not be resold or incorporated in any manner into another product for resale. Any use for therapeutics or diagnostics is strictly prohibited. This product is covered by U.S. Patent(s), pending patent applications and foreign equivalents. - Excitation Laser
-
Violet Laser (405 nm)
- Application Notes
-
Additional reported applications (for the relevant formats) include: immunoprecipitation1,2, complement-dependent cytotoxicity3, in vivo depletion4,5,9,10, mediation of in vitro redirected lysis6, blocking of NK cell function7, induction of proliferation8, immunohistochemical staining of frozen sections11, immunofluorescence microscopy11, and spatial biology (IBEX)16,17. The LEAF™ purified antibody (Endotoxin <0.1 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for functional assays (Cat. No. 108712).
- Additional Product Notes
-
Iterative Bleaching Extended multi-pleXity (IBEX) is a fluorescent imaging technique capable of highly-multiplexed spatial analysis. The method relies on cyclical bleaching of panels of fluorescent antibodies in order to image and analyze many markers over multiple cycles of staining, imaging, and, bleaching. It is a community-developed open-access method developed by the Center for Advanced Tissue Imaging (CAT-I) in the National Institute of Allergy and Infectious Diseases (NIAID, NIH).
-
Application References
(PubMed link indicates BioLegend citation) -
- Carlyle JR, et al. 1999. J. Immunol. 162:5917. (IP)
- Sentman CL, et al. 1989. Hybridoma 8:605. (IP)
- Koo GC, et al. 1984. Hybridoma 3:301. (Cyt)
- Sentman CL, et al. 1989. J. Immunol. 142:1847. (Deplete)
- Koo GC, et al. 1986. J. Immunol. 137:3742. (Deplete)
- Karlhofer FM, et al. 1991. J. Immunol. 146:3662.
- Kung SK, et al. 1999. J. Immunol. 162:5876. (Block)
- Reichlin A, et al. 1998. Immunol. Cell Biol. 76:143.
- Drobyski W, et al. 1996. Blood 87:5355. (Deplete)
- Andoniou CE, et al. 2005. Nat. Immunol. 6:1011. (Deplete)
- Kanwar JR, et al. 2001. J. Natl. Cancer Inst. 93:1541. (IHC, IF)
- Kroemer A, et al. 2008. J. Immunol. 180:7818. PubMed
- Kim JY, et al. 2009. Exp Mol Med. 30:288. PubMed
- Bankoti J, et al. 2010. Toxicol. Sci. 115:422. (FC) PubMed
- Lee H, et al. 2014. Invest Ophthalmol Vis Sci. 55:2885. PubMed
- Radtke AJ, et al. 2020. Proc Natl Acad Sci U S A. 117:33455-65. (SB) PubMed
- Radtke AJ, et al. 2022. Nat Protoc. 17:378-401. (SB) PubMed
- Product Citations
- RRID
-
AB_10895916 (BioLegend Cat. No. 108731)
AB_2562561 (BioLegend Cat. No. 108741)
AB_2562218 (BioLegend Cat. No. 108732)
Antigen Details
- Structure
- NKR-P1 gene family
- Distribution
-
NK and NK-T cells in the NK1.1 mouse strains (C57BL, FVB/N, NZB)
- Function
- NK cell activation, IFN-γ production, cytotoxic granule release
- Cell Type
- NK cells, NKT cells
- Biology Area
- Immunology, Innate Immunity
- Antigen References
-
1. Lanier LL. 1997. Immunity 6:371.
2. Yokoyama WM, et al. 1993. Ann. Rev. Immunol. 11:613.
3. Koo GC, et al. 1986. J. Immunol. 137:3742.
4. Giorda R, et al. 1991. J. Immunol. 147:1701. - Gene ID
- 17059 View all products for this Gene ID
- UniProt
- View information about NK-1.1 on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- What is the F/P ratio range of our BV421™ format antibody reagents?
-
It is lot-specific. On average it ranges between 2-4.
- If an antibody clone has been previously successfully used in IBEX in one fluorescent format, will other antibody formats work as well?
-
It’s likely that other fluorophore conjugates to the same antibody clone will also be compatible with IBEX using the same sample fixation procedure. Ultimately a directly conjugated antibody’s utility in fluorescent imaging and IBEX may be specific to the sample and microscope being used in the experiment. Some antibody clone conjugates may perform better than others due to performance differences in non-specific binding, fluorophore brightness, and other biochemical properties unique to that conjugate.
- Will antibodies my lab is already using for fluorescent or chromogenic IHC work in IBEX?
-
Fundamentally, IBEX as a technique that works much in the same way as single antibody panels or single marker IF/IHC. If you’re already successfully using an antibody clone on a sample of interest, it is likely that clone will have utility in IBEX. It is expected some optimization and testing of different antibody fluorophore conjugates will be required to find a suitable format; however, legacy microscopy techniques like chromogenic IHC on fixed or frozen tissue is an excellent place to start looking for useful antibodies.
- Are other fluorophores compatible with IBEX?
-
Over 18 fluorescent formats have been screened for use in IBEX, however, it is likely that other fluorophores are able to be rapidly bleached in IBEX. If a fluorophore format is already suitable for your imaging platform it can be tested for compatibility in IBEX.
- The same antibody works in one tissue type but not another. What is happening?
-
Differences in tissue properties may impact both the ability of an antibody to bind its target specifically and impact the ability of a specific fluorophore conjugate to overcome the background fluorescent signal in a given tissue. Secondary stains, as well as testing multiple fluorescent conjugates of the same clone, may help to troubleshoot challenging targets or tissues. Using a reference control tissue may also give confidence in the specificity of your staining.
- How can I be sure the staining I’m seeing in my tissue is real?
-
In general, best practices for validating an antibody in traditional chromogenic or fluorescent IHC are applicable to IBEX. Please reference the Nature Methods review on antibody based multiplexed imaging for resources on validating antibodies for IBEX.
Customers Also Purchased
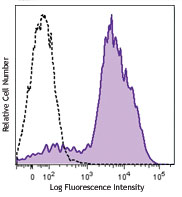
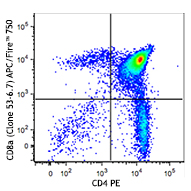
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
 Login / Register
Login / Register 













Follow Us