- Clone
- H1.2F3 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- Very Early Activation Antigen (VEA), AIM, EA1, MLR3, gp34/28
- Isotype
- Armenian Hamster IgG
- Ave. Rating
- Submit a Review
- Product Citations
- publications
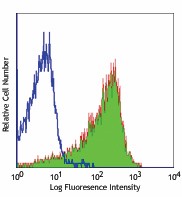
-

Con A-stimulated Balb/c mouse splenocytes (24 hours) stained with purified H1.2F3, followed by anti-Armenian hamster IgG FITC
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 104502 | 500 µg | 203€ | ||||
CD69 is a 60 kD type II membrane protein composed of a 27/33 kD disulfide-linked homodimer, also known as Very Early Activation Antigen (VEA), AIM, EA1, MLR3, and gp34/28. It is expressed on a subset of thymocytes and platelets. CD69 is rapidly induced on activated T and B cells, neutrophils, and NK cells. It is a C-type lectin, closely related to the NKR-P1 and Ly-49 NK cell activation molecules. CD69 is involved in the early events of cell activation and thymocyte positive selection.
Product DetailsProduct Details
- Reactivity
- Mouse
- Antibody Type
- Monoclonal
- Host Species
- Armenian Hamster
- Immunogen
- Mouse dendritic epidermal T cell line Y245
- Formulation
- Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide.
- Preparation
- The antibody was purified by affinity chromatography.
- Concentration
- 0.5 mg/ml
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C.
- Application
-
FC - Quality tested
IHC-F, IP - Reported in the literature, not verified in house - Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 1.0 µg per million cells in 100 µl volume. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
The H1.2F3 antibody has been reported to augment T cell activation. Additional reported applications (for the relevant formats) include: in vitro T cell and NK cell activation1-3, immunohistochemistry4,5, and immunoprecipitation1.
This antibody has been characterized in the literature as containing a lambda (?) light chain. - Application References
-
- Yokoyama WM, et al. 1988. J. Immunol. 141:369. (IP)
- Sobel ES, et al. 1993. J. Immunol. 150:673.
- Karlhofer FM, et al. 1991. J. Immunol. 146:3662.
- Zhou X, et al. 2005. J. Biol. Chem. 280:31240. (IHC)
- Podd BS, et al. 2006. J. Immunol. 176:6532. (IHC)
- Lawson BR, et al. 2007. J. Immunol. 178:5366.
- Lee JW, et al. 2006. Nature Immunol. 8:181.
- Epardaud M, et al. 2008. Cancer Res. 15:2972. PubMed
- Jordan JM, et al. 2008. 76:3717. PubMed
- Kenna TJ, et al. 2008. Blood 111:2091. PubMed
- Ishikawa C, et al. 2013. Biochim Biophys Acta. 167:99. PubMed
- Product Citations
- RRID
-
AB_313105 (BioLegend Cat. No. 104502)
Antigen Details
- Structure
- C-type lectin, 27/33 kD
- Distribution
-
Activated T cells and B cells, NK cells, granulocytes, thymocytes, platelets
- Function
- Lymphocyte activation
- Cell Type
- B cells, Granulocytes, NK cells, Platelets, T cells, Thymocytes, Tregs
- Biology Area
- Costimulatory Molecules, Immunology, Innate Immunity
- Molecular Family
- CD Molecules
- Antigen References
-
1. Barclay AN, et al. 1997. The Leukocyte Antigen FactsBook Academic Press.
2. Testi R, et al. 1994. Immunol. Today 15:479.
3. Moretta A, et al. 1991. J. Exp. Med. 174:1393.
4. Yokoyama WM, et al. 1988. J. Immunol. 141:369. - Gene ID
- 12515 View all products for this Gene ID
- UniProt
- View information about CD69 on UniProt.org
Related FAQs
Customers Also Purchased
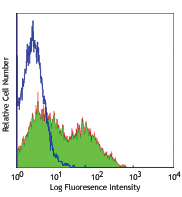
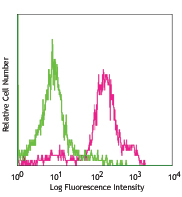
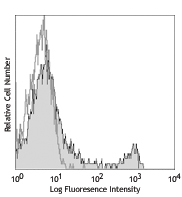

Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
 Login / Register
Login / Register 








Follow Us