- Other Names
- Chemically-defined Low DMSO Cryopreservation Solution
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
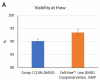
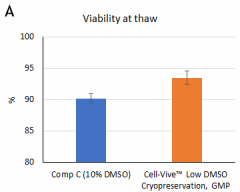
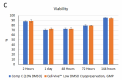
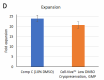
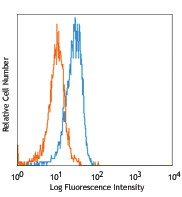
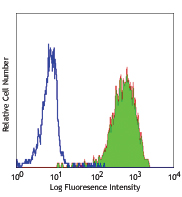
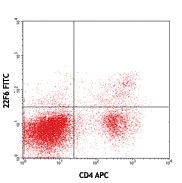
Cell-Vive™ Low DMSO Cryopreservation, GMP preserves T cell viability during post-cryopreservation expansion, at similar or higher levels than other commonly used solutions containing 10% DMSO (denoted as Comp C in the figures). After a 9-day expansion protocol using anti-human CD28, anti-human CD3, and IL-2, activated and expanded T cells were cryopreserved and thawed. Viability (A) and cell recovery (B) (% of cells recovered versus the initial number frozen) were determined at thaw. Viability was assessed by Trypan blue; cell recovery was assessed using an automatized cell counter. Cells were re-activated with 1 µg/mL of Ultra-LEAF™ anti-human CD3 antibody (Cat. No. 317347), 1 µg/mL of Ultra-LEAF™ anti-human CD28 antibody (Cat. No. 302943), and viability was determined again at several time points post-thaw (C). Expansion ability was assessed after a 6-day (144 hours) culture period. The expansion fold was calculated by dividing the number of viable cells at day 6 by the number of viable cells at 2h post-thaw (D). Flow cytometry was used to quantify CD4+ and CD8+ T cell populations at thaw, after the above 9-day expansion protocol (E). -

-
-
-
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 420504 | 50 mL | 165 CHF | ||||
Cell-Vive™ Low DMSO Cryopreservation, GMP is specifically formulated to support immune cell cryopreservation, while making use of low DMSO content. It is a chemically-defined formulation, prepared without bovine or other animal components. It is suitable for use in the cryopreservation of several T cell sources, without affecting relevant T cell properties. When used under the appropriate conditions, this product can preserve T cell viability and expansion capacity. This GMP product is suggested for use in research and for further manufacturing applications. The benefits of this cryopreservation solution include:
- Low DMSO content.
- Chemically-defined formulation.
- Effective in cryopreserving T cells from multiple sources (PBMCs, CD3+, expanded T cells).
- Able to maintain T cell properties after cryopreservation.
- Minimizes safety risks associated with DMSO.
- Improved lot-to-lot consistency.
BioLegend Cell-Vive™ GMP cell culture products are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include
- Low endotoxin level (< 1 EU/mL)
- Mycoplasma and bacterial/fungal growth testing
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- QA review of released products
- Quality audits per ISO 13485:2016
Product Details
- Formulation
- Chemically-defined formulation
- Endotoxin Level
- < 1EU/mL
- Storage & Handling
- Store at 2°C to 8°C.
- Application
-
Cell Culture
- Application Notes
-
For cryopreservation, a range between 5-50 million cells per 1 mL of Cell-Vive™ CD Low DMSO Cryopreservation, GMP is recommended. The appearance of the product in liquid form is clear with a slight yellow color.
Cryopreservation protocol- Prepare an immune cells suspension following an appropriate protocol.
- Centrifuge cells at 300 x g for 5 minutes to obtain a pellet. Carefully decant or aspirate supernatant
- Resuspend pelleted cells with Cell-Vive™ CD Low DMSO Cryopreservation, GMP and dispense the cell suspension into a cryovial.
- Immediately transfer the cryovial into a cell freezing container and store it at -70°C
- After 24-36 hours, transfer cryovials into a liquid nitrogen tank for long-term storage.
Thawing cells protocol
- Thaw cryovials containing cells by putting them in a 37°C water bath with very gently swirling. Thaw until only a small ice fragment is present.
- In a biosafety hood, add 1 mL of preferred cell culture media such as IMDM into the vial and then transfer content into a 15 mL conical tube containing 8mL of cell culture media at room temperature. Gently mix
- Centrifuge cells at 300 x g for 5 minutes. Carefully decant or aspirate supernatant
- Resuspend the cells at the desired density with cell culture media.
- Disclaimer
-
BioLegend Cell-Vive™ GMP Cell Culture products are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products.
Antigen Details
- Gene ID
- NA
 Login / Register
Login / Register 














Follow Us