- Clone
- 30-F11 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- T200, Ly-5, LCA
- Isotype
- Rat IgG2b, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
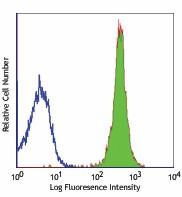
-

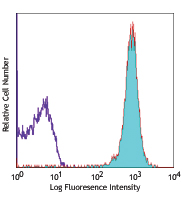
C57BL/6 mouse splenocytes stained with 30-F11 Alexa Fluor® 488 -
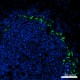
Confocal image of C57BL/6 mouse spleen sample acquired using the IBEX method of highly multiplexed antibody-based imaging: CD169 (green) in Cycle 2 and CD45 (blue) in Cycle 3. Tissues were prepared using ~1% (vol/vol) formaldehyde and a detergent. Following fixation, samples are immersed in 30% (wt/vol) sucrose for cryoprotection. Images are courtesy of Drs. Andrea J. Radtke and Ronald N. Germain of the Center for Advanced Tissue Imaging (CAT-I) in the National Institute of Allergy and Infectious Diseases (NIAID, NIH).
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 103121 | 25 µg | 81€ | ||||
| 103122 | 100 µg | 184€ | ||||
CD45 is a 180-240 kD glycoprotein also known as the leukocyte common antigen (LCA), T200, or Ly-5. It is a member of the protein tyrosine phosphatase (PTP) family, expressed on all hematopoietic cells except mature erythrocytes and platelets. There are different isoforms of CD45 that arise from variable splicing of exons 4, 5, and 6, which encode A, B, and C determinants, respectively. CD45 plays a key role in TCR and BCR signal transduction. These isoforms are very specific to the activation and maturation state of the cell as well as cell type. The primary ligands for CD45 are galectin-1, CD2, CD3, CD4, TCR, CD22, and Thy-1.
Product DetailsProduct Details
- Reactivity
- Mouse
- Antibody Type
- Monoclonal
- Host Species
- Rat
- Immunogen
- Mouse thymus or spleen
- Formulation
- Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide.
- Preparation
- The antibody was purified by affinity chromatography and conjugated with Alexa Fluor® 488 under optimal conditions.
- Concentration
- 0.5 mg/mL
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C, and protected from prolonged exposure to light. Do not freeze.
- Application
-
FC - Quality tested
SB - Reported in the literature, not verified in house
- Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 0.25 µg per 106 cells in 100 µL volume. It is recommended that the reagent be titrated for optimal performance for each application.
* Alexa Fluor® 488 has a maximum emission of 519 nm when it is excited at 488 nm.
Alexa Fluor® and Pacific Blue™ are trademarks of Life Technologies Corporation.
View full statement regarding label licenses - Excitation Laser
-
Blue Laser (488 nm)
- Application Notes
-
Clone 30-F11 reacts with all isoforms and both CD45.1 and CD45.2 alloantigens of CD45.
Additional reported applications (for relevant formats) include: immunoprecipitation3, complement-dependent cytotoxicity1,5, immunohistochemistry (acetone-fixed frozen sections, zinc-fixed paraffin-embedded sections and formalin-fixed paraffin-embedded sections)4,6, Western blotting7, and spatial biology (IBEX)10,11. The Ultra-LEAF™ purified antibody (Endotoxin < 0.01 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for functional assays (Cat. No. 103163 and 103164). - Additional Product Notes
-
Iterative Bleaching Extended multi-pleXity (IBEX) is a fluorescent imaging technique capable of highly-multiplexed spatial analysis. The method relies on cyclical bleaching of panels of fluorescent antibodies in order to image and analyze many markers over multiple cycles of staining, imaging, and, bleaching. It is a community-developed open-access method developed by the Center for Advanced Tissue Imaging (CAT-I) in the National Institute of Allergy and Infectious Diseases (NIAID, NIH).
-
Application References
(PubMed link indicates BioLegend citation) -
- Podd BS, et al. 2006. J. Immunol. 176:6532. (FC, CMCD) PubMed
- Haynes NM, et al. 2007. J. Immunol. 179:5099. (FC)
- Ledbetter JA, et al. 1979. Immunol. Rev. 47:63. (IP)
- Simon DI, et al. 2000. J. Clin. Invest. 105:293. (IHC)
- Seaman WE. 1983. J. Immunol. 130:1713. (CMCD)
- Cornet A, et al. 2001. P. Natl. Acad. Sci. USA 98:13306. (IHC)
- Tsuboi S and Fukuda M. 1998. J. Biol. Chem. 273:30680. (WB) PubMed
- Liu F, et al. 2012. Blood. 119:3295. PubMed
- Pelletier AN, et al. 2012. J. Immunol. 188:5561. PubMed
- Radtke AJ, et al. 2020. Proc Natl Acad Sci U S A. 117:33455-65. (SB) PubMed
- Radtke AJ, et al. 2022. Nat Protoc. 17:378-401. (SB) PubMed
- Product Citations
- RRID
-
AB_493532 (BioLegend Cat. No. 103121)
AB_493531 (BioLegend Cat. No. 103122)
Antigen Details
- Structure
- Protein tyrosine phosphatase (PTP) family, 180-240 kD
- Distribution
-
All hematopoietic cells except mature erythrocytes and platelets
- Function
- Phosphatase, T and B cell activation
- Ligand/Receptor
- Galectin-1, CD2, CD3, CD4, TCR, CD22, Thy-1
- Cell Type
- B cells, Dendritic cells, Mesenchymal Stem Cells, Tregs
- Biology Area
- Cell Biology, Immunology, Inhibitory Molecules, Innate Immunity, Neuroscience, Neuroscience Cell Markers, Stem Cells
- Molecular Family
- CD Molecules
- Antigen References
-
1. Barclay A, et al. 1997. The Leukocyte Antigen FactsBook Academic Press.
2. Trowbridge IS, et al. 1993. Annu. Rev. Immunol. 12:85.
3. Kishihara K, et al. 1993. Cell 74:143.
4. Pulido R, et al. 1988. J. Immunol. 140:3851. - Gene ID
- 19264 View all products for this Gene ID
- UniProt
- View information about CD45 on UniProt.org
Related FAQs
- If an antibody clone has been previously successfully used in IBEX in one fluorescent format, will other antibody formats work as well?
-
It’s likely that other fluorophore conjugates to the same antibody clone will also be compatible with IBEX using the same sample fixation procedure. Ultimately a directly conjugated antibody’s utility in fluorescent imaging and IBEX may be specific to the sample and microscope being used in the experiment. Some antibody clone conjugates may perform better than others due to performance differences in non-specific binding, fluorophore brightness, and other biochemical properties unique to that conjugate.
- Will antibodies my lab is already using for fluorescent or chromogenic IHC work in IBEX?
-
Fundamentally, IBEX as a technique that works much in the same way as single antibody panels or single marker IF/IHC. If you’re already successfully using an antibody clone on a sample of interest, it is likely that clone will have utility in IBEX. It is expected some optimization and testing of different antibody fluorophore conjugates will be required to find a suitable format; however, legacy microscopy techniques like chromogenic IHC on fixed or frozen tissue is an excellent place to start looking for useful antibodies.
- Are other fluorophores compatible with IBEX?
-
Over 18 fluorescent formats have been screened for use in IBEX, however, it is likely that other fluorophores are able to be rapidly bleached in IBEX. If a fluorophore format is already suitable for your imaging platform it can be tested for compatibility in IBEX.
- The same antibody works in one tissue type but not another. What is happening?
-
Differences in tissue properties may impact both the ability of an antibody to bind its target specifically and impact the ability of a specific fluorophore conjugate to overcome the background fluorescent signal in a given tissue. Secondary stains, as well as testing multiple fluorescent conjugates of the same clone, may help to troubleshoot challenging targets or tissues. Using a reference control tissue may also give confidence in the specificity of your staining.
- How can I be sure the staining I’m seeing in my tissue is real?
-
In general, best practices for validating an antibody in traditional chromogenic or fluorescent IHC are applicable to IBEX. Please reference the Nature Methods review on antibody based multiplexed imaging for resources on validating antibodies for IBEX.
Customers Also Purchased
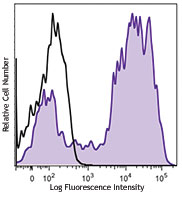
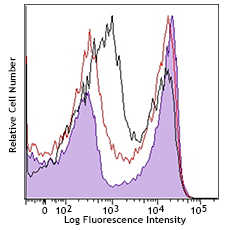
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
 Login / Register
Login / Register 














Follow Us