- Clone
- UCHT1 (See other available formats)
- Regulatory Status
- RUO
- Workshop
- III 471
- Other Names
- T3, CD3ε
- Isotype
- Mouse IgG1, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
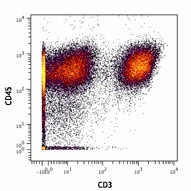
-

Human PBMCs stained with 154Sm-anti-CD45 (HI30) and 170Er-anti-CD3 (UCHT1). Data provided by DVS Sciences.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 300443 | 100 µg | 104€ | ||||
CD3ε is a 20 kD chain of the CD3/T-cell receptor (TCR) complex which is composed of two CD3ε, one CD3γ, one CD3δ, one CD3ζ (CD247), and a T-cell receptor (α/β or γ/δ) heterodimer. It is found on all mature T cells, NKT cells, and some thymocytes. CD3, also known as T3, is a member of the immunoglobulin superfamily that plays a role in antigen recognition, signal transduction, and T cell activation.
Product DetailsProduct Details
- Reactivity
- Human
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide and EDTA.
- Preparation
- The antibody was purified by affinity chromatography.
- Concentration
- 1.0 mg/ml
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C.
- Application
-
FC - Quality tested
CyTOF®, PG - Verified - Recommended Usage
-
This product is suitable for use with the Maxpar® Metal Labeling Kits. For metal labeling using Maxpar® Ready antibodies, proceed directly to the step to Partially Reduce the Antibody by adding 100 µl of Maxpar® Ready antibody to 100 µl of 4 mM TCEP-R in a 50 kDa filter and continue with the protocol. Always refer to the latest version of Maxpar® User Guide when conjugating Maxpar® Ready antibodies.
- Application Notes
-
Additional reported applications (for the relevant formats) include: immunohistochemical staining of acetone-fixed frozen sections4,6,7 and formalin-fixed paraffin-embedded sections11, immunoprecipitation1, activation of T cells2,3,5, Western blotting9, and spatial biology (IBEX)16,17. The LEAF™ purified antibody (Endotoxin < 0.1 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for functional assays (Cat. No. 300413, 300414, and 300432). For highly sensitive assays, we recommend Ultra-LEAF™ purified antibody (Cat. No. 300437, 300438, 300465, 300466, 300473, 300474) with a lower endotoxin limit than standard LEAF™ purified antibodies (Endotoxin < 0.01 EU/µg).
- Additional Product Notes
-
Maxpar® is a registered trademark of Standard BioTools Inc.
- Application References
-
- Salmeron A, et al. 1991. J. Immunol. 147:3047. (IP)
- Graves J, et al. 1991. J. Immunol. 146:2102. (Activ)
- Lafont V, et al. 2000. J. Biol. Chem. 275:19282. (Activ)
- Ryschich E, et al. 2003. Tissue Antigens 62:48. (IHC)
- Thompson AG, et al. 2004. J. Immunol. 173:1671. (Activ)
- Sakkas LI, et al. 1998. Clin. Diagn. Lab. Immun. 5:430. (IHC)
- Mack CL, et al. 2004. Pediatr. Res. 56:79. (IHC)
- Thakral D, et al. 2008. J. Immunol. 180:7431. (FC) PubMed
- Van Dongen JJM, et al. 1988. Blood 71:603. (WB)
- Yoshino N, et al. 2000. Exp. Anim. (Tokyo) 49:97. (FC)
- Pollard, K. et al. 1987. J. Histochem. Cytochem. 35:1329. (IHC)
- Luckashenak N, et al. 2013. J. Immunol. 190:27. PubMed
- Laurent AJ, et al. 2014. PLoS One. 9:103683. PubMed
- Li J, et al. 2015. Cancer Res. 75:508. PubMed
- Stoeckius M, et al. 2017. Nat. Methods. 14:865-868. (PG)
- Radtke AJ, et al. 2020. Proc Natl Acad Sci USA. 117:33455-33465. (SB) PubMed
- Radtke AJ, et al. 2022. Nat Protoc. 17:378-401. (SB) PubMed
- Product Citations
- RRID
-
AB_2562808 (BioLegend Cat. No. 300443)
Antigen Details
- Structure
- Ig superfamily, with the subunits of CD3γ, CD3δ, CD3ζ (CD247) and TCR (α/β or γ/δ) forms CD3/TCR complex, 20 kD
- Distribution
-
Mature T and NK T cells, thymocyte differentiation
- Function
- Antigen recognition, signal transduction, T cell activation
- Ligand/Receptor
- Peptide antigen bound to MHC
- Cell Type
- NKT cells, T cells, Thymocytes, Tregs
- Biology Area
- Immunology, Innate Immunity
- Molecular Family
- CD Molecules, TCRs
- Antigen References
-
1. Barclay N, et al. 1993. The Leucocyte FactsBook. Academic Press. San Diego.
2. Beverly P, et al. 1981. Eur. J. Immunol. 11:329.
3. Lanier L, et al. 1986. J. Immunol. 137:2501-2507. - Gene ID
- 916 View all products for this Gene ID
- UniProt
- View information about CD3 on UniProt.org
Related FAQs
- Can I obtain CyTOF data related to your Maxpar® Ready antibody clones?
-
We do not test our antibodies by mass cytometry or on a CyTOF machine in-house. The data displayed on our website is provided by Fluidigm®. Please contact Fluidigm® directly for additional data and further details.
- Can I use Maxpar® Ready format clones for flow cytometry staining?
-
We have not tested the Maxpar® Ready antibodies formulated in solution containing EDTA for flow cytometry staining. While it is likely that this will work in majority of the situations, it is best to use the non-EDTA formulated version of the same clone for flow cytometry testing. The presence of EDTA in some situations might negatively affect staining.
- I am having difficulty observing a signal after conjugating a metal tag to your Maxpar® antibody. Please help troubleshoot.
-
We only supply the antibody and not test that in house. Please contact Fluidigm® directly for troubleshooting advice: http://techsupport.fluidigm.com/
- Is there a difference between buffer formulations related to Maxpar® Ready and purified format antibodies?
-
The Maxpar® Ready format antibody clones are formulated in Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide and EDTA. The regular purified format clones are formulated in solution that does not contain any EDTA. Both formulations are however without any extra carrier proteins.
Customers Also Purchased
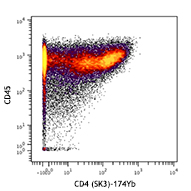
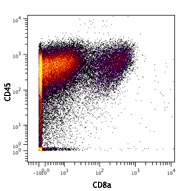
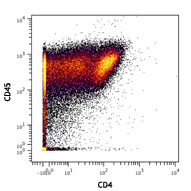
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
 Login / Register
Login / Register 














Follow Us