- Clone
- SMI 32 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- Neurofilament heavy polypeptide, NF-H, 200 kD neurofilament protein, neurofilament triplet H protein
- Previously
-
Covance Catalog# SMI-32P
- Isotype
- Mouse IgG1
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

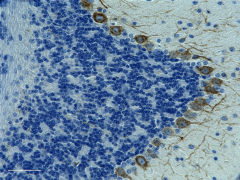
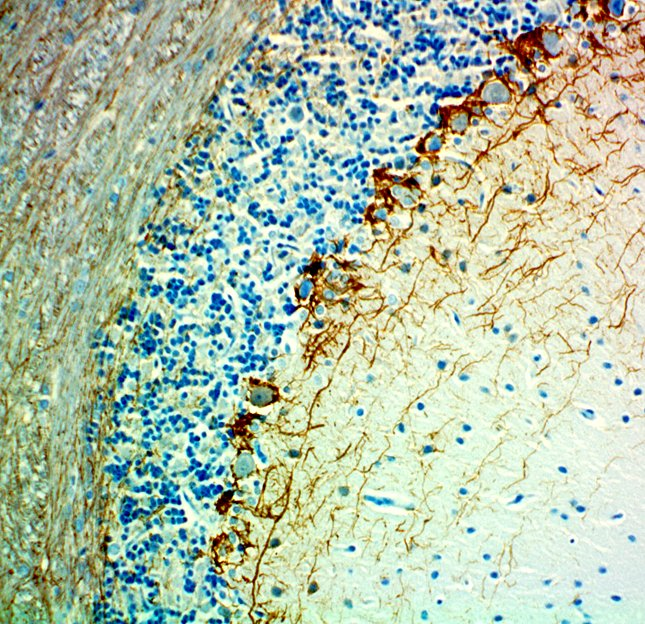
IHC staining of purified anti-Neurofilament H (NF-H), Nonphosphorylated antibody (clone SMI 32) on formalin-fixed paraffin-embedded mouse brain tissue. Following antigen retrieval using Retrieval-ALL Antigen Unmasking System 3 (Cat. No. 927601), the tissue was incubated with 1 µg/ml of the primary antibody overnight at 4°C. BioLegend’s Ultra Streptavidin (USA) HRP Detection Kit (Multi-Species, DAB, Cat. No. 929901) was used for detection followed by hematoxylin counterstaining, according to the protocol provided. The image was captured with a 40X objective. Scale bar: 50µm -
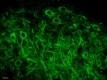
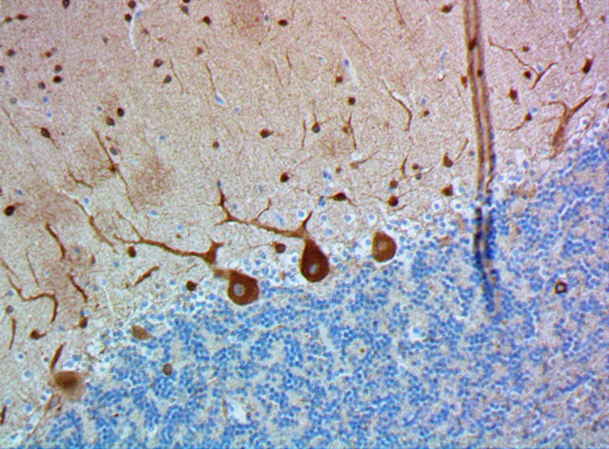
Immunofluorescence staining of anti-Neurofilament H (NF-H), Nonphosphorylated antibody (clone SMI 32) on formalin-fixed paraffin-embedded (FFPE) tissue section from mouse Thalamus. After antigen retrieval using Retrieval-ALL Antigen Unmasking System 3 (Cat. No. 927601), the tissue was blocked with Normal Serum Block for 30 min at room temperature, and then incubated with SMI 32 at 5 µg/mL overnight at 4°C, followed by incubation with Alexa Fluor® 488 Goat anti-mouse IgG (Cat. No. 405319) for one hour at room temperature. The image was captured with a 40X objective (Scale Bar: 20 µm). -
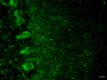
IHC-Fluorescence staining of Formalin Fixed Paraffin Embedded (FFPE) Rat Cerebellum by anti-Neurofilament H (NF-H), Nonphosphorylated Antibody clone SMI 32. After antigen retrieval using Retrieval-ALL Antigen Unmasking System 3 (Cat. No. 927601), the tissue was blocked with Normal Serum Block for 30 min at room temperature, and then incubated with SMI 32 at 5 µg/mL overnight at 4°C, followed by incubation with Alexa Fluor® 488 Goat anti-mouse IgG (Cat. No. 405319) for one hour at room temperature. The image was captured with a 40X objective (Scale Bar: 20 µm). -
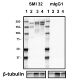
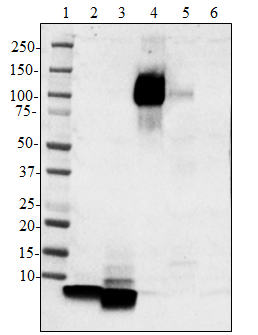
Western blot of purified anti-Neurofilament H (NF-H), Nonphosphorylated antibody (clone SMI 32). Lane 1: Molecular weight marker; Lane 2: 20 µg of human brain lysate; Lane 3: 20 µg of mouse brain lysate; Lane 4: 20 µg of rat brain lysate. The blot was incubated with 1 ug/mL of the primary antibody overnight at 4°C, followed by incubation with HRP-labeled goat anti-mouse IgG (Cat. No. 405306). Enhanced chemiluminescence was used as the detection system.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 801702 | 25 µL | 90€ | ||||
| 801701 | 100 µL | 221€ | ||||
Neurofilaments (NF) are approximately 10 nanometer intermediate filaments found in neurons. They are a major component of the neuronal cytoskeleton, and function primarily to provide structural support for the axon and to regulate the axon diameter. There are three major NF subunits, and the names given to these subunits are based upon the apparent molecular mass of the mammalian subunits on SDS-PAGE. The light or lowest NF (NF-L) runs at 68-70 kD. The medium or middle NF (NF-M) runs at about 145-160 kD, and the heavy or highest NF (NF-H) runs at 200-220 kD. However, the actual molecular weight of these proteins is considerably lower due to the highly charged C-terminal regions of the molecules. The level of NF gene expression correlates with the axonal diameter, which controls how fast electrical signals travel down the axon. Mutant mice with NF abnormalities have phenotypes resembling amyotrophic lateral sclerosis. NF immunostaining is common in diagnostic neuropathology. It is useful for differentiating neurons (positive for NF) from the glia (negative for NF).
Product DetailsProduct Details
- Reactivity
- Human,Mouse,Rat
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- Phosphate-buffered solution (no preservatives or carrier proteins).
- Preparation
- The antibody was purified by affinity chromatography.
- Concentration
- 1 mg/ml
- Storage & Handling
- This antibody should be handled aseptically as it is free of preservatives such as Sodium Azide. Store this antibody undiluted between 2°C and 8°C. Please note the storage condition for this antibody has been changed from -20°C to between 2°C and 8°C. You can also check the vial label or CoA to find the proper storage conditions.
- Application
-
IHC-P - Quality tested
WB - Verified
Array Tomography, ICC - Reported in the literature, not verified in house
SB - Community verified - Recommended Usage
-
Each lot of this antibody is quality control tested by formalin-fixed paraffin-embedded immunohistochemical staining. For immunohistochemistry, a concentration range of 1.0 - 5.0 µg/ml is suggested. For Western blotting, the suggested use of this reagent is 1.0 - 5.0 µg per ml. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
Additional reported applications (for the relevant formats) include Western blotting6, immunohistochemistry4,5, immunocytochemistry1,2,3, 7, array tomography8.
Cross-reactivity to monkey tissue has been Reported in the literature, not verified in house4.
This antibody reacts with a nonphosphorylated epitope in neurofilament H of most mammalian species. The reaction is masked when the epitope is phosphorylated. The staining of isolated neurofilament preparations is greatly intensified upon dephosphorylation. Immunocytochemically, SMI 32 visualizes neuronal cell bodies, dendrites, and some thick axons in the central and peripheral nervous systems. However, thin axons are not revealed. Other cells and tissues are unreactive. The antibody distinguishes three subdivisions of the macaque precentral motor cortex. The greater size of the left versus the right superior temporal lobe was found to be due to increased axonal myelination and not due to increased number of glial cells or SMI 32-enumerated neurons, suggesting that the specialization for language in the left temporal lobe is related to increased speed of signal transmission. In cultures of murine cortex, SMI 32 labels a neuronal population with enhanced vulnerability to kainate toxicity most of which are GABAergic and reveal kainate-activated Ca2+ uptake. - Additional Product Notes
-
This product has been verified for IHC-P (Immunohistochemistry - formalin-fixed paraffin-embedded tissues) on the NanoString GeoMx® Digital Spatial Profiler. The GeoMx® enables researchers to perform spatial analysis of protein and RNA targets in FFPE and fresh frozen human and mouse samples. For more information about our spatial biology products and the GeoMx® platform, please visit our spatial biology page.
- Application References
-
- Chang Q, Martin LJ. 2011. J. Neurosci., 31:2815-27. (ICC) PubMed
- Stevens HE, et al. 2010. J. Neurosci. 30:5590-602. (ICC) PubMed
- Kiryu-Seo S, et al. 2010. J. Neurosci. 30:6658-66. (ICC) PubMed
- Redondo J, et al. 2015. Brain Pathol. 25(6):692. (IHC-P) PubMed
- Feng L, et al. 2017. eNeuro. 4(1): 0331-16.2016. (IHC-P) PubMed
- Feng L, et al. 2014. Invest Ophthalmol Vis Sci. 54(2): 1106–1117. (IHC-P) PubMed
- Theotokis, et al. 2016. J. Neuroinflammation 13(1):265 (IHC-P)
- Bennett, et al. 2015. J. Neurosci. Methods 245:25-36 (Array Tomography)
- Petzold A, et al. 2011. Brain 134:464. (WB) PubMed
- Product Citations
- RRID
-
AB_2715852 (BioLegend Cat. No. 801702)
AB_2564642 (BioLegend Cat. No. 801701)
Antigen Details
- Structure
- Neurofilament H has an apparent molecular mass of 200-220 kD.
- Distribution
-
Tissue distribution: CNS, peripheral nerves and glandular cells of the prostate
Cellular distribution: Cytoskeleton, nucleus, cytosol, and mitochondrion - Function
- NF-H Neurofilaments are the major components of the neuronal cytoskeleton. They provide axonal support and regulate axon diameter. Phosphorylation of NF-H results in the formation of interfilament cross bridges that are important in the maintenance of axonal caliber.
- Receptors
- Phosphorylation seems to play a major role in the functioning of the larger neurofilament polypeptides (NF-M and NF-H), the levels of phosphorylation result in changes to the neurofilament function.
- Cell Type
- Mature Neurons
- Biology Area
- Cell Biology, Neuroscience, Neuroscience Cell Markers
- Molecular Family
- Intermediate Filaments, Phospho-Proteins
- Antigen References
-
- Turner M, et al. 2015. Journal of Neuroimmunology. 285: 4. PubMed
- Pagliarini V, et al. 2015. J. Cell Biol.. 211: 77. PubMed
- Petzold A, et al. 2011. Brain 134. (WB) PubMed
- Yuan A, et al. 2016. Brain Res Bull 126(3): 334.
- Parlakian A, et al. 2016. Rev Neurol. 172(10): 607.
- Li D, et al. 2016. Front Aging Neurosci. 8: 290.
- Costa J, et al. 2016. Clin Chim Acta. 455: 7.
- Lad SP, et al. 2010. J Stroke Cerebrovasc Dis. 21(1): 30.
- Gene ID
- 4744 View all products for this Gene ID
- UniProt
- View information about Neurofilament H on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- If an antibody clone has been previously successfully used in IBEX in one fluorescent format, will other antibody formats work as well?
-
It’s likely that other fluorophore conjugates to the same antibody clone will also be compatible with IBEX using the same sample fixation procedure. Ultimately a directly conjugated antibody’s utility in fluorescent imaging and IBEX may be specific to the sample and microscope being used in the experiment. Some antibody clone conjugates may perform better than others due to performance differences in non-specific binding, fluorophore brightness, and other biochemical properties unique to that conjugate.
- Will antibodies my lab is already using for fluorescent or chromogenic IHC work in IBEX?
-
Fundamentally, IBEX as a technique that works much in the same way as single antibody panels or single marker IF/IHC. If you’re already successfully using an antibody clone on a sample of interest, it is likely that clone will have utility in IBEX. It is expected some optimization and testing of different antibody fluorophore conjugates will be required to find a suitable format; however, legacy microscopy techniques like chromogenic IHC on fixed or frozen tissue is an excellent place to start looking for useful antibodies.
- Are other fluorophores compatible with IBEX?
-
Over 18 fluorescent formats have been screened for use in IBEX, however, it is likely that other fluorophores are able to be rapidly bleached in IBEX. If a fluorophore format is already suitable for your imaging platform it can be tested for compatibility in IBEX.
- The same antibody works in one tissue type but not another. What is happening?
-
Differences in tissue properties may impact both the ability of an antibody to bind its target specifically and impact the ability of a specific fluorophore conjugate to overcome the background fluorescent signal in a given tissue. Secondary stains, as well as testing multiple fluorescent conjugates of the same clone, may help to troubleshoot challenging targets or tissues. Using a reference control tissue may also give confidence in the specificity of your staining.
- How can I be sure the staining I’m seeing in my tissue is real?
-
In general, best practices for validating an antibody in traditional chromogenic or fluorescent IHC are applicable to IBEX. Please reference the Nature Methods review on antibody based multiplexed imaging for resources on validating antibodies for IBEX.
Other Formats
View All Neurofilament H (NF-H), Nonphosphorylated Reagents Request Custom ConjugationCustomers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
 Login / Register
Login / Register 













Follow Us