- Clone
- M1/70 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- αM integrin, Mac-1, Mo1, CR3, Ly-40, C3biR, ITGAM
- Isotype
- Rat IgG2b, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
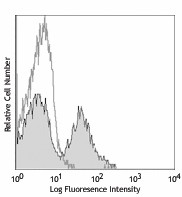
-

C57BL/6 mouse bone marrow cells were stained with Ultra-LEAF™ purified CD11b (clone M1/70) (filled histogram) or rat IgG2b, κ isotype control (open histogram), followed by anti-rat IgG FITC (gated on total cells).
Select size of product is eligible for a 40% discount! Promotion valid until September 30, 2024. Exclusions apply. To view full promotion terms and conditions or to contact your local BioLegend representative to receive a quote, visit our webpage.
CD11b is a 170 kD glycoprotein also known as αM integrin, Mac-1 α subunit, Mol, CR3, and Ly-40. CD11b is a member of the integrin family, primarily expressed on granulocytes, monocytes/macrophages, dendritic cells, NK cells, and subsets of T and B cells. CD11b non-covalently associates with CD18 (β2 integrin) to form Mac-1. Mac-1 plays an important role in cell-cell interaction by binding its ligands ICAM-1 (CD54), ICAM-2 (CD102), ICAM-4 (CD242), iC3b, and fibrinogen.
Product DetailsProduct Details
- Reactivity
- Mouse,Human,Cynomolgus,Rhesus
- Antibody Type
- Monoclonal
- Host Species
- Rat
- Immunogen
- C57BL/10 splenocytes
- Formulation
- 0.2 µm filtered in phosphate-buffered solution, pH 7.2, containing no preservative.
- Preparation
- The Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody was purified by affinity chromatography.
- Concentration
- The antibody is bottled at the concentration indicated on the vial, typically between 2 mg/mL and 3 mg/mL. Older lots may have also been bottled at 1 mg/mL. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
FC - Quality tested
CyTOF® - Verified
IP, Block, Depletion, IHC-F, ICC - Reported in the literature, not verified in house - Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤0.25 µg per million cells in 100 µl volume or 100 µl of whole blood. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
Clone M1/70 has been verified for immunocytochemistry (ICC) and frozen immunohistochemistry (IHC-F).
Additional reported applications (for relevant formats of this clone) include: immunoprecipitation1,4, in vitro blocking3,9,12, depletion2,8, immunofluorescence microscopy6,7,10, immunohistochemistry of acetone-fixed frozen sections5,11-13, and spatial biology (IBEX)35,36. For in vivo studies or highly sensitive assays, we recommend Ultra-LEAF™ purified antibody (Endotoxin < 0.01 EU/µg, Azide-Free, 0.2 µm filtered) (Cat. No. 101248). -
Application References
(PubMed link indicates BioLegend citation) -
- Springer T, et al. 1978. Eur. J. Immunol. 8:539. (IP)
- Ault K and Springer T. 1981. J. Immunol. 126:359. (Deplete)
- Springer TA, et al. 1982. Immunol. Rev. 68:171. (Block)
- Ho MK and Springer TA. 1983. J. Biol. Chem. 258:2766. (IP)
- Flotte TJ, et al. 1983. Am. J. Pathol. 111:112. (IHC)
- Noel GJ, et al. 1990. J. Clin. Invest. 85:208. (IF)
- Allen LA and Aderem A. 1996. J. Exp. Med. 184:627 (IF)
- D'Amico A and Wu L. 2003. J. Exp. Med. 198:293. (Deplete)
- Brickson SJ, et al. 2003. Appl Physiol. 95:969. (Block)
- Clatworthy MR and Smith KG. 2004. J. Exp. Med. 199:717. (IF)
- Hata H, et al. 2004. J. Clin. Invest. 114:582. (IHC)
- Zhang Y, et al. 2002. J. Immunol. 168:3088. (IHC)
- Iwasaki A and Kelsall BL. 2001. J. Immunol. 166:4884 (IHC, FC)
- Tailleux L. 2003. J. Exp. Med. 197:121. (Block, FC)
- Olver S, et al. 2006. Cancer Research 66:571. (FC)
- Tan SL, et al. 2006. J. Immunol. 176:2872. (FC) PubMed
- Ponomarev ED, et al. 2006. J. Immunol. 176:1402. (FC)
- Dzhagalov I, et al. 2007. Blood 109:1620. (FC)
- Fazilleau N, et al. 2007. Nature Immunol. 8:753.
- Rasmussen JW, et al. 2006. Infect. Immun.74:6590. PubMed
- Napimoga MH, et al. 2008. J. Immunol. 180:609. PubMed
- Elqaraz-Carmon V, et al. 2008. J. Lipid. Res. 49:1894. PubMed
- Kim DD, et al. 2008. Blood 112:1109. PubMed
- Guo Y, et al. 2008. Blood 112:480. PubMed
- Norian LA, et al. 2009. Cancer Res. 69:3086. (FC) PubMed
- Baumgartner CK, et al. 2010. J. Immunol. 184:573. PubMed
- Charles N, et al. 2010. Nat. Med. 16:701. (FC) PubMed
- Whiteland J, et al. 1995. J. Histochem. Cytochem. 43:313. (IHC)
- Weber GF, et al. 2014. J Exp Med. 211:1243. PubMed
- Ashok A, et al. 2015. Toxicol Sci. 143:64. PubMed
- Price PJ, et al. 2015. J Immunol. 194:1164. PubMed
- Doni A, et al. 2015. J Exp Med. 212:905. PubMed
- Ferreira R, et al. 2016. J Infect Dis. 213: 669 - 673. PubMed
- Peterson VM, et al. 2017. Nat. Biotechnol. 35:936. (PG)
- Radtke AJ, et al. 2020. Proc Natl Acad Sci U S A. 117:33455-65. (SB) PubMed
- Radtke AJ, et al. 2022. Nat Protoc. 17:378-401. (SB) PubMed
- Product Citations
- RRID
-
AB_2813917 (BioLegend Cat. No. 101247)
AB_2561479 (BioLegend Cat. No. 101248)
AB_2813918 (BioLegend Cat. No. 101269)
AB_2813919 (BioLegend Cat. No. 101270)
AB_2813920 (BioLegend Cat. No. 101271)
AB_2813921 (BioLegend Cat. No. 101272)
Antigen Details
- Structure
- Integrin family, associates with integrin β2 (CD18), 170 kD
- Distribution
-
Granulocytes, monocytes/macrophages, dendritic cells, NK cells, subsets of T and B cells
- Function
- Adhesion, chemotaxis
- Ligand/Receptor
- ICAM-1 (CD54), ICAM-2 (CD102), ICAM-4 (CD242), iC3b, fibrinogen
- Cell Type
- B cells, Dendritic cells, Granulocytes, Macrophages, Monocytes, Neutrophils, NK cells, T cells, Tregs
- Biology Area
- Cell Adhesion, Cell Biology, Costimulatory Molecules, Immunology, Innate Immunity, Neuroscience, Neuroscience Cell Markers
- Molecular Family
- Adhesion Molecules, CD Molecules
- Antigen References
-
1. Barclay A, et al. 1997. The Leukocyte Antigen FactsBook Academic Press.
2. Springer TA. 1994. Cell 76:301.
3. Coxon A, et al. 1996. Immunity 5:653. - Gene ID
- 16409 View all products for this Gene ID 3684 View all products for this Gene ID
- UniProt
- View information about CD11b on UniProt.org
Related FAQs
- Do you guarantee that your antibodies are totally pathogen free?
-
BioLegend does not test for pathogens in-house aside from the GoInVivo™ product line. However, upon request, this can be tested on a custom basis with an outside, independent laboratory.
- Does BioLegend test each Ultra-LEAF™ antibody by functional assay?
-
No, BioLegend does not test Ultra-LEAF™ antibodies by functional assays unless otherwise indicated. Due to the possible complexities and variations of uses of biofunctional antibodies in different assays and because of the large product portfolio, BioLegend does not currently perform functional assays as a routine QC for the antibodies. However, we do provide references in which the antibodies were used for functional assays and we do perform QC to verify the specificity and quality of the antibody based on our strict specification criteria.
- Does BioLegend test each Ultra-LEAF™ antibody for potential pathogens?
-
No, BioLegend does not test for pathogens in-house unless otherwise indicated. However, we can recommend an outside vendor to perform this testing as needed.
- Have you tested this Ultra-LEAF™ antibody for in vivo or in vitro applications?
-
We don't test our antibodies for in vivo or in vitro applications unless otherwise indicated. Depending on the product, the TDS may describe literature supporting usage of a particular product for bioassay. It may be best to further consult the literature to find clone specific information.
Customers Also Purchased
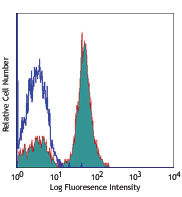
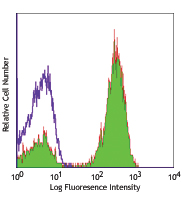
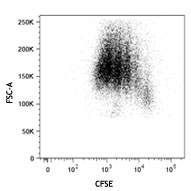
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.











Follow Us