- Clone
- 581 (See other available formats)
- Other Names
- Gp105-120, My10
- Isotype
- Mouse IgG1, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

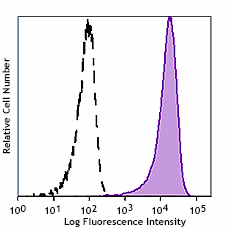
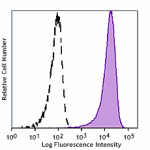
Human myeloid cell line KG1a stained with Cell-Vive™ GMP Ultra-LEAF™ anti-human CD34 SF antibody (Clone 581, filled histogram) or isotype control (Clone MOPC-21, open histogram) followed by FITC anti-mouse IgG stain.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 343547 | 100 µg | 308€ | ||||
| 343548 | 1 mg | 1584€ | ||||
CD34, also known as gp105-120, is a type I monomeric sialomucin-like glycophosphoprotein with an approximate molecular weight of 105-120 kD. Selectively expressed on the majority of hematopoietic stem/progenitor cells, bone marrow stromal cells, capillary endothelial cells, embryonic fibroblasts, and some nervous tissue, CD34 is a commonly used marker to identify human hematopoietic stem/progenitor cells. According to the differential sensitivity to enzymatic cleavage, four groups of epitopes of CD34 have been described. CD34 mediates cell adhesion and lymphocytes homing through binding to L-selectin and E-selectin ligands.
Cell-Vive™ GMP Ultra-LEAF™ Purified anti-human CD34 SF Antibody was GMP manufactured under serum-free conditions including serum-free hybridoma cell culture, without additional animal or human-derived materials or preservatives. This antibody contains ultra-low levels of endotoxin (<0.01 EU/μg of protein), is filtered through a 0.1 μm membrane, and is tested negative for mycoplasma and microbial growths.
Cell-Vive™ GMP Ultra-LEAF™ Purified anti-human CD34 SF Antibody is for research and further ex vivo cell processing use only.
BioLegend Cell-Vive™ GMP Ultra-LEAF™ antibodies are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include:
- Ultra-low endotoxin level (<0.01 EU/µg)
- Manufactured under serum-free conditions
- Bulks tested negative for mycoplasma and bacterial/fungal growth
- Serum-free hybridomas are negative for the presence of murine virus
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- QA review of released products
- Quality audits per ISO 13485:2016
Product Details
- Reactivity
- Human
- Reported Reactivity
- Cynomolgus
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Formulation
- 0.1 μm filtered in phosphate-buffered solution, pH 7.2, containing no preservative.
- Endotoxin Level
- < 0.01 EU/μg of the protein (< 0.001 ng/μg of the protein) as determined by the LAL test.
- Preparation
- Serum-Free hybridoma derived Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody purified by affinity chromatography.
- Concentration
- 1.0 mg/mL
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
FC - Quality tested
ICC, IHC-P, CyTOF® - Reported in the literature - Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 2.0 µg per million cells in 100 µL volume. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
Cell-Vive™ GMP Ultra-LEAF™ Purified anti-human CD34 SF Antibody (Endotoxin < 0.01 EU/μg, Azide-Free, 0.1 μm filtered) is recommended for highly sensitive assays. The antibody was GMP manufactured under serum-free conditions including serum-free hybridoma cell culture, without additional animal or human-derived materials or preservatives. This antibody is intended for flow cytometry and for research or further ex vivo cell processing use only.
The 581 clone recognizes the class III group epitope which is resistant to sialidase/glycolyprotease and chymopapain treatment. Additional reported applications (for the relevant formats) include: immunohistochemical staining of paraffin-embedded tissue sections5 and immunofluorescence6. - Application References
-
- Schlossman SF, et al. 1995. Leukocyte Typing V: White Cell Differentiation Antigen. New York: Oxford University Press.
- Felschow DM, et al. 2001. Blood. 97:3768.
- Rudin CE, et al. 1997. Br. J. Haematol. 97:488.
- Yoshino N, et al. 2000. Exp. Anim. (Tokyo) 49:97. (FC)
- Skowasch D, et al. 2003. Cardiovasc Res. 60:684. (IHC)
- Umland O, et al. 2003. J. Histochem. Cytochem. 51:977. (IF)
- Disclaimer
-
BioLegend Cell-Vive™ GMP Ultra-LEAF™ antibodies are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products.
Antigen Details
- Structure
- 105-120 kD single chain mucin-like glycoprotein
- Distribution
-
Hematopoietic stem/progenitor cells, bone marrow stromal cells, endothelial cells, embryonic fibroblasts
- Function
- Cell adhesion
- Interaction
- Endothelial cells, Fibroblasts, Hematopoietic stem, and progenitors
- Ligand/Receptor
- L-selectin, E-selectin
- Antigen References
-
- Krause DS, et al. 1996. Blood. 87:1.
- Puri KD, et al. 1995. J. Cell Biol. 131:261.
- Zola H, et al. 2007. Leukocyte and Stromal Cell Molecules: The CD Markers. John Wiley & Sons Inc, Hoboken New Jersey.
- Gene ID
- 947 View all products for this Gene ID
- UniProt
- View information about CD34 on UniProt.org
Related Pages & Pathways
Pages
Other Formats
View All CD34 Reagents Request Custom ConjugationCompare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Purified anti-human CD34
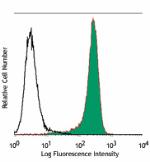
Human myeloid cell line KG1a stained with purified 581, foll... -
FITC anti-human CD34
Human peripheral blood mononuclear cells stained with 581 FI... 
-
PE anti-human CD34

Human peripheral blood mononuclear cells stained with 581 PE... 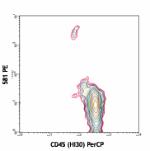
-
Alexa Fluor® 647 anti-human CD34
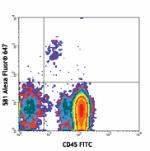
Human peripheral blood leukocytes stained with 581 Alexa Flu... 
-
APC anti-human CD34
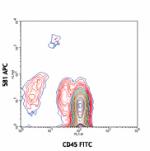
Human peripheral blood leukocytes stained with 581 APC/CD45 ... 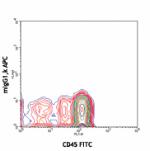
-
Pacific Blue™ anti-human CD34
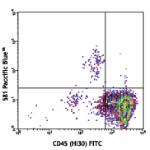
Human peripheral blood leukocytes stained with CD34 Pacific... 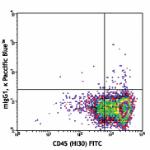
-
APC/Cyanine7 anti-human CD34

Human peripheral blood mononuclear cells were stained with C... -
PE/Cyanine7 anti-human CD34
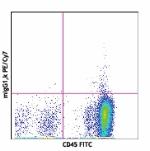
Human peripheral blood stained with 581 PE/Cyanine7 or mouse... 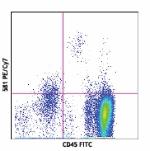
-
Alexa Fluor® 488 anti-human CD34
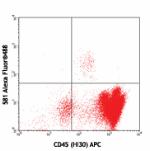
Human peripheral blood leukocytes stained with CD45 (HI30) A... 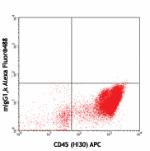
-
PerCP anti-human CD34
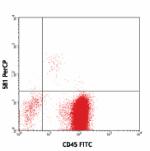
Human peripheral blood leukocytes stained with CD45 FITC and... 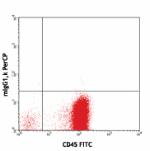
-
PerCP/Cyanine5.5 anti-human CD34

Human peripheral blood was stained with CD45 APC and CD34 (C... -
Biotin anti-human CD34
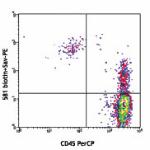
Human peripheral blood lymphocytes stained with biotinylated... 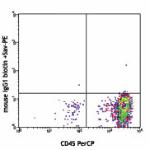
-
Alexa Fluor® 700 anti-human CD34
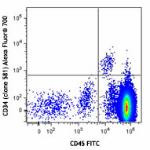
Human peripheral blood mononuclear cells were stained with C... 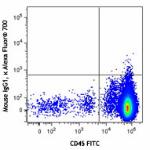
-
Brilliant Violet 510™ anti-human CD34
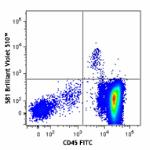
Human peripheral blood mononuclear cells were stained with C... 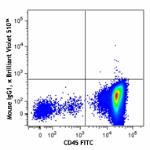
-
Purified anti-human CD34 (Maxpar® Ready)
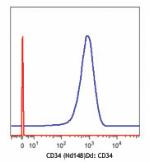
Human KG-1a cells (blue) and mouse EL4 T cells (red) stained... -
PE/Dazzle™ 594 anti-human CD34
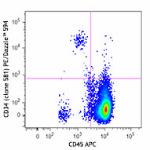
Human peripheral blood leukocytes were stained with CD14 Bri... 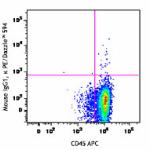
-
APC/Fire™ 750 anti-human CD34
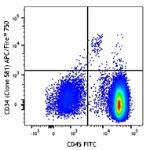
Human peripheral blood mononuclear cells were stained with C... 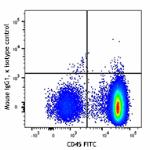
-
TotalSeq™-A0054 anti-human CD34
-
TotalSeq™-B0054 anti-human CD34
-
TotalSeq™-C0054 anti-human CD34
-
TotalSeq™-D0054 anti-human CD34
-
Spark Red™ 718 anti-human CD34
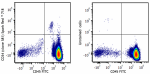
Human peripheral blood leukocytes were stained with anti-hum... -
Cell-Vive™ GMP Ultra-LEAF™ Purified anti-human CD34 SF
Human myeloid cell line KG1a stained with Cell-Vive™ GMP Ult... -
Cell-Vive™ GMP Ultra-LEAF™ Biotin anti-human CD34 SF Antibody

Human myeloid cell line KG-1a stained with Cell-Vive™ GMP Ul... -
Spark PLUS UV™ 395 anti-human CD34

Human peripheral blood leukocytes were stained with anti-hum...
 Login / Register
Login / Register 









Follow Us